Therapeutics Development for COVID-19 and Viral Diseases
Research Tools and Resources

There is an urgent need to develop therapeutic approaches to limit the spread and impact of the SARS-CoV-2 virus. Efforts are directed both toward qualifying existing drugs as safe and efficacious to use in the context of COVID-19 and developing novel therapeutic agents that specifically target SARS-CoV-2.
Pharmaceutical companies have ramped up their work to identify and generate monoclonal antibodies (mAbs) that can be used to neutralize SARS-CoV-2. Cellular therapy, successful in the treatment of diseases such as cancer, may also have applications in the treatment of COVID-19 or its long-lasting effects. Regardless of the class of therapy being developed, researchers are reliant upon in vitro models to provide predictive insight into the magnitude and mechanism of both therapeutic effect and possible toxicity.
Explore technologies, applications, and research tools for developing therapeutics to combat viral diseases.
Therapeutic Antibody Generation

Monoclonal antibodies (mAbs) that target specific protein epitopes are promising therapies for a variety of diseases, including viral infection. Antibody therapeutics specific to viral proteins can neutralize the virus by binding to it and preventing it from entering host cells and replicating.
The efficacy of therapeutic antibodies for COVID-19 specifically is supported by results of the use of convalescent plasma in critical patients.1, 2 Multiple strategies, including generating hybridomas and immortalizing primary human B cells, can be used to develop mAb antiviral therapies against SARS-CoV-2.
Using Hybridomas for Monoclonal Antibody Generation
Hybridomas are often generated from the fusion of myeloma cells and the B cells of wild-type or humanized mice as an early step in therapeutic monoclonal antibody development. This step allows for isolation of single antibody sequences that recognize and bind to the target antigen with high affinity.
The efficiency of hybridoma generation can be enhanced by: increasing the number of productive fusions between splenocytes and myeloma cells; decreasing the resources needed to generate a library of unique clones; increasing the diversity of clones isolated; and increasing the survival and expansion capacity of hybridoma clones under stressful conditions. Prior to fusion, isolating B cells with EasySep™ Mouse CD138 Positive Selection Kit can dramatically increase the efficiency of hybridoma fusion, while ClonaCell™-HY media provide robust growth conditions for fusion, cloning, and hybridoma expansion (Figure 1).
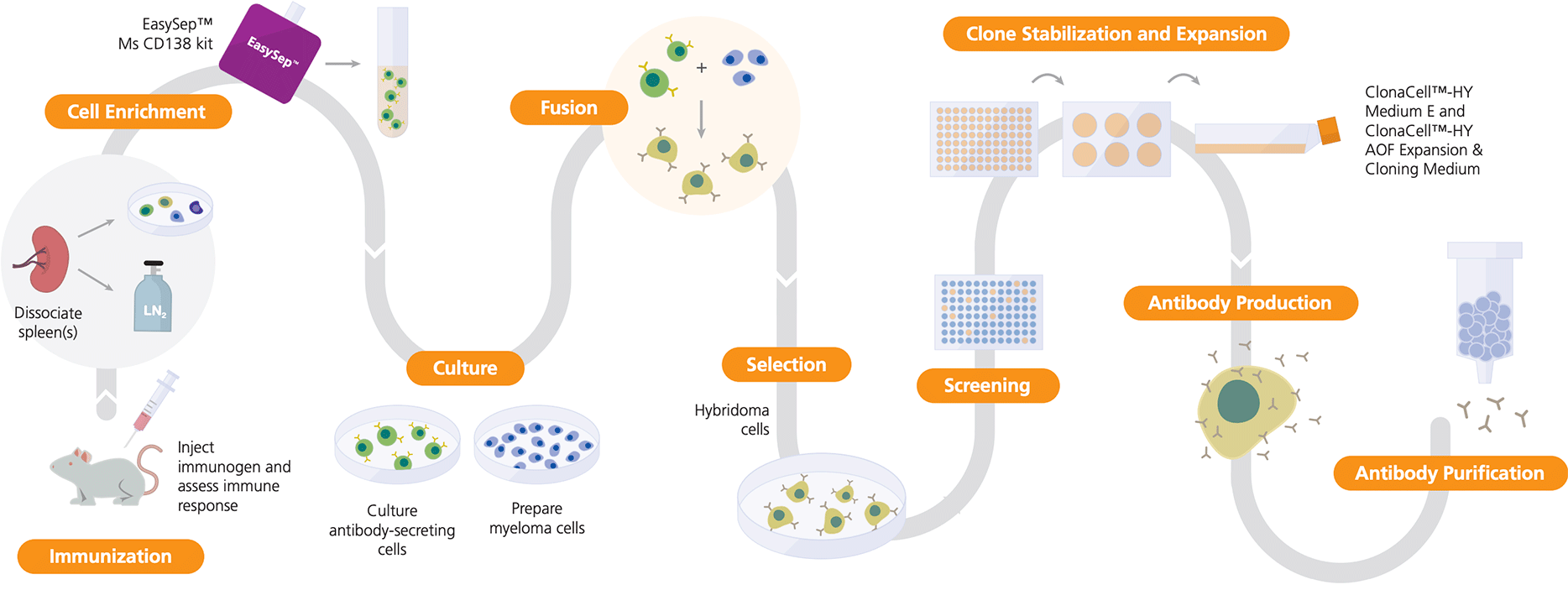
Figure 1. Hybridoma Workflow Using ClonaCell™ and EasySep™
The dissociated spleen (or other lymphoid tissue) from an immunized animal is used in fusion reactions with myeloma cells to generate hybridomas. EasySep™ Mouse CD138 Kit enriches for antibody-secreting cells prior to fusion. Hybridomas are selected for antibody secretion and target specificity, and the desired clones are stabilized over time using ClonaCell™-HY Medium E or ClonaCell™-HY AOF Expansion Medium.
Products for Hybridoma Generation
Studies of SARS-CoV with ClonaCell™
Nieto-Torres JL et al. (2011) Subcellular location and topology of severe acute respiratory syndrome coronavirus envelope protein. Virology 415(2): 69–82.
Researchers generated mAbs to study the subcellular localization of a SARS-CoV protein during infection. They supported hybridoma clone survival and expansion with ClonaCell™-HY Medium E.
Lip KM et al. (2006) Monoclonal antibodies targeting the HR2 domain and the region immediately upstream of the HR2 of the S protein neutralize in vitro infection of severe acute respiratory syndrome coronavirus. J Virol 80(2): 941–50.
Researchers used the ClonaCell™-HY Kit (for steps from pre-fusion culture to hybridoma expansion) to generate monoclonal hybridomas using the semi-solid cloning method. They studied the interactions between the mAbs made by these hybridomas and purified viral protein.
Berry JD et al. (2004) Development and characterisation of neutralising monoclonal antibody to the SARS-coronavirus. J Virol Methods 120(1): 87–96.
To generate mAbs that recognize the spike proteins of SARS-CoV, a research group in Canada used semi-solid cloning in ClonaCell™- HY Medium D to simultaneously select for successful hybridoma fusion and isolate individual hybridoma clones. Two of the spike-specific mAbs demonstrated the ability to neutralize SARS-CoV in vitro.
Antibody Generation with Primary Human B Cells
An efficient alternative to generating hybridomas is immortalizing primary human B cells.3 Although the speed and efficiency of this approach depends on the screening and transfection methods employed, using techniques like transient transfection can reduce the timeframe for assessing the initial viral neutralization potential of candidate recombinant mAbs to one week. Researchers have successfully employed this approach to obtain neutralizing antibodies against SARS-CoV from immortalized memory B cells obtained from recovered patients.4 Naïve B cell populations can also be used to generate potent virus-neutralizing human mAbs. These can be expanded using ImmunoCult™-ACF Human B Cell Expansion Supplement. You can further streamline mAb generation by using EasySep™, a smarter, more efficient way to isolate cells, and by choosing a trusted supplier for human primary cells.
Products for Antibody Generation with Primary Human B Cells
*For information about COVID-19 and primary cells, please see our frequently asked questions (FAQ). Certain products are only available in select territories.
- For fully automated cell separations from up to 16 samples
Antibody Production in CHO Cells
To manufacture antibody therapeutics, mAbs need to be produced as recombinant proteins in expression systems with a proven safety profile, such as Chinese hamster ovary (CHO) cells. To support cell survival and expansion during cloning and other vulnerable stages of cell line development, use ClonaCell™-CHO ACF Supplement, which is compatible with a wide variety of workflows and platforms.
Products for Antibody Production in CHO Cells
Immunoassays
In early development phases, mAb-producing cell lines are assessed to identify high-producing lines. Researchers often rely on highly sensitive enzyme-linked immunosorbent assays (ELISAs) to ensure accurate quantification of mAbs.
Products to Quantify mAbs
Convalescent Plasma Therapy Research
In this classical adaptive immunotherapy, convalescent plasma from recovered patients is used to treat patients with viral infections. Lacking an established therapeutic approach for COVID-19, physicians are investigating convalescent plasma therapy as an option in critical cases, with promising initial results.1,2
To obtain the antibodies from a recovered patient, scientists need to isolate plasma from individual blood samples. Patient blood samples can be processed quickly and consistently by using SepMate™ and Lymphoprep™ for streamlined density gradient centrifugation, where samples can be rapidly added to tubes and the top layer easily pipetted off.
Cellular Therapy Research
Immunotherapy, which strengthens the body's own immune system to fight disease, is another approach under investigation for the treatment of viral diseases.
NK Cell-Based Therapy Research
Natural killer (NK) cell-based therapies harness the ability of NK cells to target and lyse antibody-bound infected cells through an immune response process called antibody-dependent cellular cytotoxicity (ADCC). Recent research has shown that NK cells can exert antiviral activity via ADCC against SARS-CoV-2.5 You can use pre-isolated, ethically sourced human NK cells, or isolate them yourself from human blood products using EasySep™. Alternatively, CD56+ NK cells can be generated from cord blood-derived CD34+ cells or differentiated from human pluripotent stem cells (hPSCs).
Products for NK Cell-Based Therapy Research
- For fully automated cell separations
*For information about COVID-19 and primary cells, please see our frequently asked questions (FAQ). Certain products are only available in select territories.
T Cell Therapy Research
T cell therapy is also under investigation as a treatment approach for viral diseases, including COVID-19.6 This form of immunotherapy uses specially altered T cells to target and kill infected cells. You can isolate T cells for cell therapy research from ethically sourced whole blood or leukapheresis products, or purchase pre-isolated cryopreserved Pan-T cells. T cells can then be expanded in vitro. Alternatively, T cells can be generated from cord blood-derived CD34+ cells or differentiated from hPSCs.
Products for T Cell Therapy Research
- For fully automated cell separations
*For information about COVID-19 and primary cells, please see our frequently asked questions (FAQ). Certain products are only available in select territories.
Hematopoietic Cell Therapeutic Development for SARS-CoV-2
There is growing interest in studying the effects of expanded cord blood-derived CD34+ cells in patients with COVID-19. These cells may have an effect on inflammation associated with infection. You can isolate CD34+ cells using EasySep™, culture and expand them in StemSpan™-ACF Without Phenol Red, and finally test their potency by performing CFU assays in MethoCult™ H4034 Optimum.
Products for Hematopoietic Cell Therapy Research
*For information about COVID-19 and primary cells, please see our frequently asked questions (FAQ). Certain products are only available in select territories.
In Vitro Assays and Models
Researchers have the opportunity to optimize development pipelines by using relevant human model systems to assess critical characteristics such as efficacy, mechanism of action, and toxicity of therapeutics in vitro. Incorporating these experiments at strategic points during development can help save valuable time by informing decisions on the best candidates to move forward.
In Vitro Human Tissue Modeling
Using physiologically relevant human model systems, researchers can gain critical, predictive results for drug screening and toxicity testing. Human in vitro models—including cultures generated by directed differentiation of hPSCs—that recapitulate characteristics at the cellular and tissue levels are being increasingly adopted at various stages during therapeutic development.
Air-liquid interface (ALI) cultures of human airway epithelial cells form pseudostratified mucociliary epithelial layers that closely model the in vivo airway, making these cultures a uniquely suited model system for studying respiratory infections in vitro. Similarly, human organoids modeling various human tissues provide in vitro model systems that incorporate multiple tissue-specific cell types and model tissue-specific cellular organization. Explore how in vitro intestinal and kidney model systems can be used to study COVID-19.
Products for In Vitro Human Tissue Modeling
Learn more about airway ALI, kidney organoid, and intestinal organoid models for COVID-19 research.
Recombinant Spike, Nucleocapsid, and Human ACE2 Protein
Products for Targeting SARS-CoV-2 Nucleoprotein and Spike Protein
Hematotoxicity Testing
Toxicity affecting blood cells can lead to serious conditions, such as anemia, neutropenia, or thrombocytopenia. Toxic effects on hematopoietic stem and progenitor cells may cause cell death, or more subtle changes in the ability of these cells to properly proliferate and differentiate to the downstream lineages that make up circulating blood.
To test for potential hematotoxicity, primary human CD34+ cells can be expanded and differentiated in vitro to specific downstream lineages using HemaTox™ Kits. Test compounds are added during the culture periods to assess their effects on the generation of specific downstream cell lineages. IC50 values for test compounds can then be determined by flow cytometry analysis.
Find efficiencies in your hematotoxicity assays by using EasySep™ to isolate hematopoietic stem and progenitor cells, and by choosing a reliable supplier for human primary cells.
Products for Hematotoxicity Testing
*For information about COVID-19 and primary cells, please see our frequently asked questions (FAQ). Certain products are only available in select territories.
Contract Assay Services

Engaging the right expertise and resources can be an effective way to create efficiencies in your drug development pipeline. Our Contract Assay Services team combines optimized media, pre-qualified cells, and specialized expertise to screen candidate therapeutics in primary cell-based assays.
If you are interested in Contract Assay Services, including toxicity testing and pulmonary services, contact us to find out how we can help you meet your goals.
Related Resources
Vaccine Development
Explore research tools and technologies used for vaccine development for prevention of viral diseases.
Hybridoma Development
Learn about hybridoma and mammalian cell line development with these technical resources.
References
- Duan K et al. (2020) Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A 117(17): 9490–6.
- Chen L et al. (2020) Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 20(4): 398–400.
- Guthmiller JJ et al. (2019) An efficient method to generate monoclonal antibodies from human B cells. Methods Mol Biol 1904:109–45.
- Traggiai E et al. (2004) An efficient method to generate monoclonal antibodies from human B cells: potent neutralization of SARS coronavirus.Nat Med 10(8):871–5.
- Zheng et al. (2020) Functional exhaustion of antiviral lymphocytes in COVID-19 patients.Cell Mol Immunol 17(5): 533–35.
- AlloVir expands its research collaboration with Baylor College of Medicine to discover and develop allogeneic, off-the-shelf, virus-specific T-cell therapies for COVID-19. Business Wire. Published online March 23, 2020.