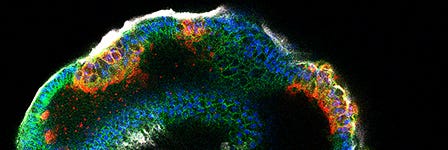
Organoid Research
One of the most exciting advancements in stem cell research of the past decade has been the development of organoid systems. Explore below for a basic overview of organoids and resources to support your organoid culture.
What Are Organoids?
Organoids are three-dimensional (3D) cell cultures that incorporate some of the key features of the represented organ. These in vitro culture systems are characterized by the self-organization of multiple, organ-specific cell types into a spatial organization similar to what is observed in vivo, and are capable of recapitulating some functions of the represented organ.1-4
How Are Organoids Made?
Organoids may be generated from adult stem cell-containing tissue samples, single adult stem cells, or via the directed differentiation of pluripotent stem cells.3,4 Due to the active stem cell population that are present in some organoid model systems, the organoids can be greatly expanded. For example, up to 1 x 106 liver organoids can be produced from a single progenitor within 5 to 6 weeks, giving researchers a highly robust and scalable platform for studying a wide range of subjects.4,5
Organoid Culture
To date, organoid culture systems have been developed to model tissue structures from all three primary cell lineages.3,4,6 Although tissue-specific culture methods vary, in general, appropriate pluripotent stem cells or tissue-specific progenitor cells are embedded in Matrigel® or another appropriate extracellular matrix. The cells are grown in the presence of cell culture media containing specific growth factors that mimic the in vivo signals required to maintain the stem cell population. Under these conditions, the embedded cells proliferate and self-organize into 3D organoid structures that, with many systems, can be passaged and maintained indefinitely.1,7 Additionally, these cultures have shown remarkable genetic stability during passaging; whole genome sequencing of liver organoids clonally expanded from a single liver progenitor cell revealed only one synonymous base substitution after three months in culture.8 To date, organoid cultures have been described for a variety of tissues, including intestinal,5,9,10 liver,11,12 pancreas,13 kidney,14 prostate,15,16 lung,17,18 optic cup,19 and brain.20
Tools and Technologies for Organoid Culture
Organoids are revolutionizing in vitro cell culture by providing accessible, physiologically relevant models that faithfully recapitulate key elements of the modeled tissue. Use these optimized media to efficiently generate organoids for your research.
Organoid Media Kits
Spend less time troubleshooting and more on experimenting with optimized organoid media kits for the growth and maintenance of organoids from primary tissue and human pluripotent stem cells.
Future Directions and References
Organoid technology holds great potential as a tool to study a wide range of subjects, including developmental biology, disease pathology, cell biology, regenerative mechanisms, precision medicine, and drug toxicity and efficacy testing. For these and other applications, organoid cultures provide highly informative, complementary approaches to the well-established 2D-culture methods and animal model systems.4,7,21,22 This technology also holds tremendous potential for regenerative medicine, as organoids present the possibility for autologous and allogeneic cell therapy through the replacement of damaged or diseased tissue with organoid-propagated tissue or stem cell populations.23,24 Such an application would allow correction of genetic abnormalities in vitro using CRISPR-Cas9 and re-introduction of the engineered healthy cells into the patient, with subsequent integration into the tissue.25,26 Patient-derived organoid cultures have also proven valuable as diagnostic tools in precision medicine applications. Organoids derived from patient samples have been used to screen patient drug responses in vitro before administering treatment to direct the care and predict therapeutic outcomes of cancer and cystic fibrosis patients.10,27,28 As the list of organoid culture systems and the techniques for their experimental exploitation grows, the utility and broad applicability of organoids continues to gain recognition across a wide range of research disciplines. The development of these culture systems represents an exciting advancement in the tools available to researchers working in basic research, and in translational and clinical contexts.
References
- Sato T & Clevers H. (2013) Growing self-organizing mini-guts from a single intestinal stem cell: Mechanism and applications. Science 340(6137): 1190–4.
- Wells JM & Spence JR. (2014) How to make an intestine. Development 141(4): 752–60.
- Lancaster MA & Knoblich JA. (2014) Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 345(6194): 1247125.
- Huch M & Koo B-K. (2015) Modeling mouse and human development using organoid cultures. Development 142(18): 3113–25.
- Spence JR et al. (2011) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470(7332): 105–9.
- Kretzschmar K & Clevers H. (2016) Organoids: Modeling development and the stem cell niche in a dish. Dev Cell 38(6): 590–600.
- Clevers H. (2016) Modeling development and disease with organoids. Cell 165(7): 1586–97.
- Huch M et al. (2015) Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160(1–2): 299–312.
- Sato T et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244): 262–5.
- Sato T et al. (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141(5): 1762–72.
- Huch M et al. (2013) In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494(7436): 247–50.
- Takebe T et al. (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499(7459): 481–4.
- Huch M et al. (2013) Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J 32(20): 2708–21.
- Takasato M et al. (2014) Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16(1): 118–26.
- Karthaus WR et al. (2014) Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159(1): 163–75.
- Gao D et al. (2014) Organoid cultures derived from patients with advanced prostate cancer. Cell 159(1): 176–87.
- Lee J-H et al. (2014) Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 156(3): 440–55.
- Dye BR et al. (2015) In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4 4: e05098.
- Eiraku M et al. (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472(7341): 51–6.
- Lancaster MA et al. (2013) Cerebral organoids model human brain development and microcephaly. Nature 501(7467): 373–9.
- McCracken KW et al. (2014) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516(7531): 400–4.
- Bigorgne AE et al. (2014) TTC7A mutations disrupt intestinal epithelial apicobasal polarity. J Clin Invest 124(1): 328–37.
- Yui S et al. (2012) Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18(4): 618–23.
- Fordham RP et al. (2013) Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13(6): 734–44.
- Dekkers JF et al. (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19(7): 939–45.
- Schwank G et al. (2013) Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13(6): 653–8.
- van de Wetering M et al. (2015) Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161(4): 933–45.
- Dekkers JF et al. (2016) Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med 8(344): 344ra84.
Scientific Resources for Organoid Research
Select the organoids you’re interested in to access technical and scientific resources to support your research, including webinars, videos, protocols, technical tips, and more.