Contract Assay Services
Contract Assay Services
STEMCELL Scientists, Working for You
Contract Assay Services is a contract research organization (CRO) established within STEMCELL Technologies that performs assay services based on in vitro and in vivo primary cell assays. We combine the power of specialized STEMCELL Technologies media and reagents with the practical knowledge of our scientists to provide both standardized and customized assay services. Choose from a portfolio of characterized assays using pre-qualified primary cells or discuss your individual needs with our in-house experts.
See Our Portfolio >Why Use Contract Assay Services?
- Our high standards for methods, materials, processes and customer communication are evident in the loyalty of our returning clientele.
- As your eyes and ears in the lab, we place a priority on communication with our clients throughout the study process.
- STEMCELL Technologies is a world leader in the development of industry-standard products for stem, progenitor and other primary cells.
- We work directly with the scientists who develop the specialized STEMCELL products used in our assays.
Service Portfolio
Since 2000, Contract Assay Services has performed such studies for over 120 pharmaceutical, biotechnology, government, and academic life science organizations worldwide. As your drug discovery partner, our in-house experts help you get the data you need with standardized assays using pre-qualified primary cells or by discussing your needs to develop customized assays.
Toxicity Testing
Measure the potential toxic effects of candidate therapeutics, including small molecule compounds and biologics.
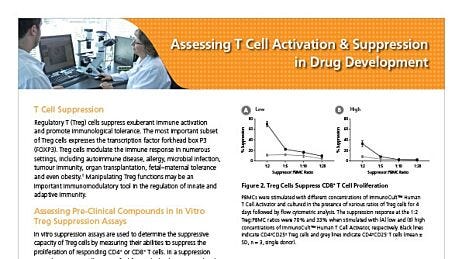
Immunological Assessment
Evaluate preclinical test compounds and biologics for their abilities to modulate the immune system.
Biopharmaceutical Testing
Examine the activity of biopharmaceutical cytokines using the CFU assay.
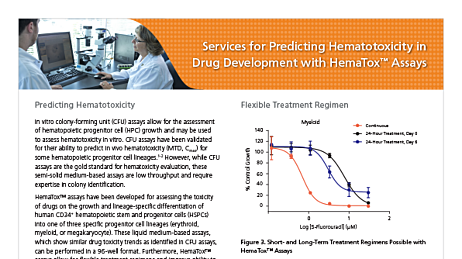
Hematopoietic Cell Services
Evaluate the effects of test compounds on hematopoietic stem cells with phenotypic and functional assessments.
Organoid-Based Assays
Supplement your data with the additional relevance of organoid-based assays.
Mesenchymal Cell Services
Characterize mesenchymal stromal cell populations for cellular and functional phenotypes.
Our Assay Services Workflow