ClonaCell™ Media for Hybridoma and CHO Cell Line Development
ClonaCell™ for Hybridoma and Cell Line Development
Liquid and Semi-Solid Media for Efficient Cell Cloning and Generation
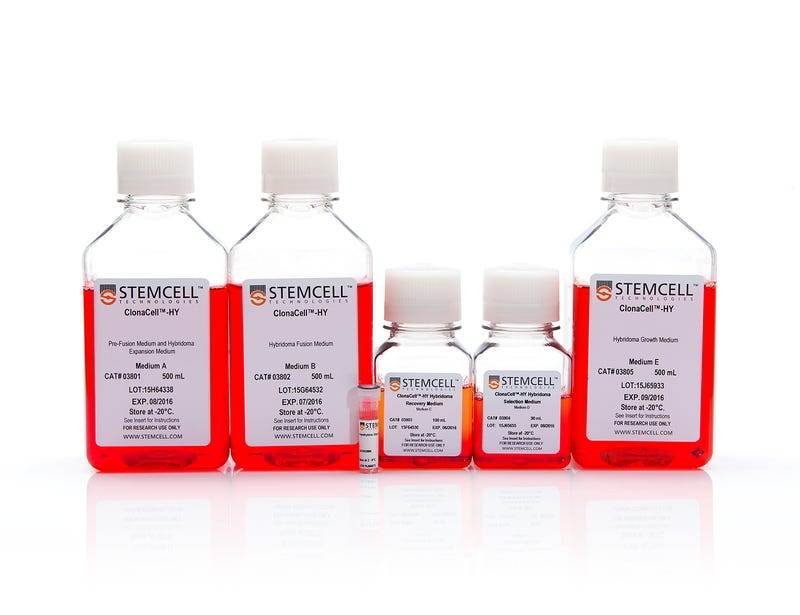
Develop CHO cell line, hybridomas, and other cell types using the ClonaCell™ portfolio of innovative cell line-specific media, designed to provide optimal culture conditions. Available in both methylcellulose-based (semi-solid) and liquid form, ClonaCell™-CHO and ClonaCell™-HY formulations help you achieve a high probability of monoclonality and clonal diversity in just one step.
View all ClonaCell™ media and supplements, and download our brochure to help you choose the right product for your needs.
Use ClonaCell™ to:
- Save time and resources with a proven semi-solid cloning method that overcomes the challenges of traditional limiting dilution cloning
- Nurture and save finicky hybridomas generated from mouse, rat, human, rabbit and hamster cells
- Increase subcloning efficiency during CHO cell line development
Research-stage biotechnology companies and antibodies service companies use ClonaCell™-HY to complete antibody development projects faster and deliver the best antibodies for each application.
Dr. M. Javad Aman, President and CSO, Integrated BioTherapeutics, Inc.
Why Use ClonaCell™?
- Save time with semi-solid cloning. Don’t waste resources on empty cloning wells.
- Superior cloning efficiency and growth of CHO and other cell lines, hybridomas and myelomas.
- Base methylcellulose medium is easily customized for your cell type. Custom formulations, packaging, and additional sizes are available upon request.
- Rigorous performance testing, ensuring lot-to-lot reproducibility.
- Industry-recognized manufacturing expertise and unparalleled technical support.
Try ClonaCell™ in your own lab.
ClonaCell™-HY Hybridoma Kit Media
Scientific Resources
Key Applications
Cell Line Development:
Rodríguez L et al. (2015) Generation of Monoclonal Antibodies Specific of The Postfusion Conformation of The Pneumovirinae Fusion (F) Protein. J Virol Methods.
Young J et al. (2015) A Novel Immunoassay to Measure Total Serum Lymphotoxin-α Levels in The Presence of An Anti-LTα Therapeutic Antibody. J Immunol Methods Epub ahead.
Chronopoulou E et al. (2014) Hybridoma Technology for The Generation of Rodent mAbs via Classical Fusion. In: Ossipow V, N Fischer (Eds.) Monoclonal Antibodies: Methods and Protocols, Methods in Molecular Biology. 2nd Ed. New York: Springer Science+Business Media.: pp47–70.
Date Y et al. (2014) Label-Free Impedimetric Immunoassay for Trace Levels of Polychlorinated Biphenyls in Insulating Oil. Anal Chem 86(6): 2989–2996.
García-Barreno B et al. (2014) Characterization of An Enhanced Antigenic Change in The Pandemic 2009 H1N1 Influenza Virus Haemagglutinin. J Gen Virol 95(PART 5): 1033–1042.
Tan GS et al. (2014) Characterization of a Broadly Neutralizing Monoclonal Antibody That Targets The Fusion Domain of Group 2 Influenza A Virus Hemagglutinin. J Virol 88(23): 13580–13592.
Kelly-Cirino CD & Mantis NJ. (2009) Neutralizing Monoclonal Antibodies Directed Against Defined Linear Epitopes on Domain 4 of Anthrax Protective Antigen. Infect Immun 77(11): 4859–4867.
Weidanz J a et al. (2006) Levels of Specific Peptide-HLA Class I Complex Predicts Tumor Cell Susceptibility to CTL Killing. J Immunol 177(8): 5088–5097.
Hybridoma Development with ClonaCell™ in Different Species:
Cesaro A et al. (2012) An Inflammation Loop Orchestrated by S100A9 and Calprotectin Is Critical for Development of Arthritis. PLoS One 7(9): e45478.
Smith S a. et al. (2012) Persistence of Circulating Memory B Cell Clones with Potential for Dengue Virus Disease Enhancement for Decades following Infection. J Virol 86(5): 2665–2675.
Fang L et al. (2008) Essential Role of TNF Receptor Superfamily 25 (TNFRSF25) in the Development of Allergic Lung Inflammation. J Exp Med 205(5): 1037–1048.
Ulbrandt ND et al. (2006) Isolation and Characterization of Monoclonal Antibodies Which Neutralize Human Metapneumovirus In Vitro and In Vivo. J Virol 80(16): 7799–7806.
Grimaldi JC et al. (1999) Depletion of Eosinophils in Mice through the Use of Antibodies Specific for C-C Chemokine Receptor 3 (CCR3). J Leukoc Biol 65(6): 846–853.
Pharmacology and Drug Discovery:
Retamal M et al. (2014) Epitope Mapping of The 2009 Pandemic and the A/Brisbane/59/2007 Seasonal (H1N1) Influenza Virus Haemagglutinins Using mAbs and Escape Mutants. J Gen Virol 95(Pt_11): 2377–2389.
Hostetter DR et al. (2007) Hip Is A Pro-Survival Substrate of Granzyme B. J Biol Chem 282(38): 27865–27874.
Jin C et al. (2006) Immunoglobulin G Specifically Binding Plant N-glycans with High Affinity Could Be Generated in Rabbits But Not in Mice. Glycobiology 16(4): 349–357.
Kuroki M et al. (2005) Preparation of Human IgG and IgM Monoclonal Antibodies for MK-1/Ep-CAM by Using Human Immunoglobulin Gene-Transferred Mouse and Gene Cloning of Their Variable Regions. Anticancer Res 25(6A): 3733–3739.
Immunology Research:
Fang L et al. (2008) Essential Role of TNF Receptor Superfamily 25 (TNFRSF25) in The Development of Allergic Lung Inflammation. J Exp Med 205(5): 1037–1048.
Matsumoto SC et al. (2006) Retinal Dysfunction in Patients with Chronic Chagas’ Disease Is Associated to Anti-Trypanosoma Cruzi Antibodies That Cross-React with Rhodopsin. FASEB J 21: 1–21.
Cancer Research:
Stern HM et al. (2010) Development of Immunohistochemistry Assays to Assess GALNT14 and FUT3/6 in Clinical Trials of Dulanermin and Drozitumab. Clin Cancer Res 16(5): 1587–1596.
Chen Y et al. (2007) Armed Antibodies Targeting The Mucin Repeats of The Ovarian Cancer Antigen, MUC16, Are Highly Efficacious in Animal Tumor Models. Cancer Res 67(10): 4924–4932.
Wittman VP et al. (2006) Antibody Targeting to A Class I MHC-Peptide Epitope Promotes Tumor Cell Death. J Immunol 177(6): 4187–4195.