Designing Your Neural Induction and Differentiation Workflow
Introduction
Neural induction is the first step in the specification of human pluripotent stem cells (hPSCs) to a neuroectodermal fate. In vivo, neural induction is initiated through the disinhibition of TGF-β and BMP signaling that occurs through the action of patterning factors released in the developing embryo1. Induction of cultured human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells to a neural fate involves mimicking these developmental cues in vitro.
This Tech Tip will walk you through practical considerations to help you select the ideal in vitro neural induction workflow to generate neural progenitor cells (NPCs), including medium choice, protocol format, and options for further expansion, cryopreservation, and differentiation of NPCs to downstream neural cell types.
Choosing a Medium: STEMdiff™ Neural Induction Medium vs. STEMdiff™ SMADi Neural Induction Kit
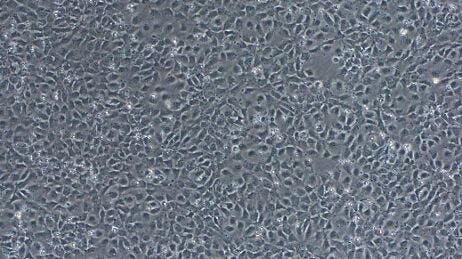
STEMdiff™ Neural Induction Medium (NIM) is a serum-free medium for the neural induction of hPSCs, including human ES and iPS cells. The cultures generated will primarily consist of central nervous system (CNS)-type NPCs, which express PAX6, SOX1, and Nestin. A smaller population of neural crest cells may also be present. If patterning factors are unwanted in your cell culture application, STEMdiff™ NIM is an appropriate choice for neural induction.
Dual SMAD inhibition (SMADi) directs efficient neural induction of hPSCs by inhibiting TGF-β- and BMP-dependent SMAD signaling2. STEMdiff™ SMADi Neural Induction Kit combines STEMdiff™ NIM with STEMdiff™ SMADi Neural Induction Supplement to significantly improve the efficiency of neural induction, even in hard-to-differentiate cell lines. Because this method results in a highly pure population of CNS-type NPCs, SMADi supplementation is strongly recommended, particularly if you will be further differentiating your NPCs to downstream cell types such as neurons or astrocytes.
Choosing a Protocol Format: Embryoid Body vs. Monolayer Protocols
Neural induction of hPSCs may be achieved using either an embryoid body (EB) or monolayer culture format, both of which are outlined in the Technical Manual for Generation and Culture of Neural Progenitor Cells Using the STEMdiff™ Neural System (Document #10000005588). Both protocol options have advantages, and either (or both) may be suitable for your research. Please refer to the table below for key considerations:
Typically results in less variability between cell lines
Typically results in less variability between replicates from the same cell line
Day 8 Checkpoint: Visual inspection of rosette formation
Compatible with hPSCs cultured in TeSR™-E8™ (SMADi highly recommended)
Compatible with hPSCs cultured in TeSR™-E8™ (SMADi highly recommended)
STEMdiff™ Midbrain Neuron Differentiation Kit (Catalog #100-0038) and STEMdiff™ Midbrain Neuron Maturation Kit (Catalog #100-0041)
STEMdiff™ Astrocyte Differentiation Kit (Catalog #100-0013) and STEMdiff™ Astrocyte Maturation Kit (Catalog #100-0016)
BrainPhys™ hPSC Neuron Kit (Catalog #05795)
- May be combined with additional patterning factors as desired to customize your neuronal differentiation
Related Protocols
Important Checkpoint: Characterization of hPSC-Derived NPCs
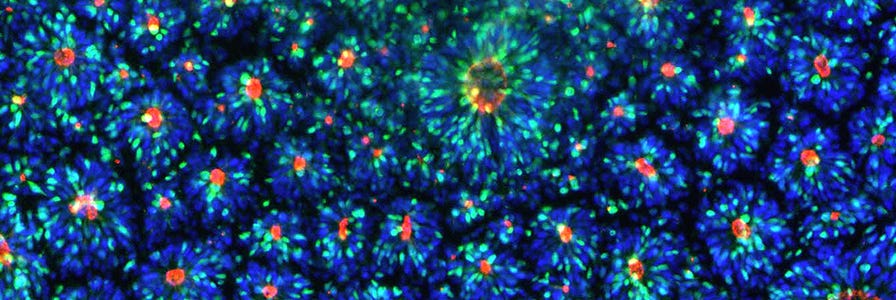
After completing the neural induction protocol, it is important to establish that the induction was successful by characterizing your resulting NPCs. Although NPC morphology can provide information about culture health, it is not a reliable method for confirming neural induction and purity. To assess the efficiency of neural induction, immunocytochemistry may be performed for the following markers to determine the percentage of cells expressing each:
*The % expression levels noted in this table are expected with conventional use of the STEMdiff™ SMADi Neural Induction Kit.
It is recommended to perform staining for at least two of the three neural markers listed above (e.g. PAX6 and SOX1, PAX6 and Nestin, or SOX1 and Nestin). Nestin should not be used on its own to evaluate induction efficiency because it may also be expressed by neural crest cells, the most common non-NPC cell type found in neural induction cultures.
For information about assessing neural/ectodermal marker expression by flow cytometry, please refer to the following Tech Tip: The STEMdiff™ Trilineage Differentiation Kit: Assessing Differentiation to Three Germ Lineages.
Increasing Workflow Yield: NPC Expansion
After neural induction, you may further maintain and expand your NPCs in STEMdiff™ Neural Progenitor Medium (NPM) for at least 10 passages with good marker expression and minimal spontaneous neuronal differentiation. NPCs will expand 3- to 5-fold per passage.
Important considerations for NPC expansion:
- If you will be performing downstream differentiation to neurons, we recommend limiting the number of NPC passages in STEMdiff™ NPM to less than five to promote the best neuronal differentiation efficiency. This is not to say that higher passage numbers won't work, but if poor efficiency is observed with high-passage NPCs, a 'younger' batch of NPCs may be worth trying.
- Periodically recharacterize neural marker expression (PAX6, SOX1, Nestin) if multiple passages have been performed before proceeding to downstream differentiation or assays.
- Ensure that at each passage, cells are seeded at the high density recommended in the manual (e.g. at least
1.25 x 105 cells/cm2). High densities are critical to maintain NPCs in a proliferative state and prevent spontaneous differentiation from occurring. - If you observe poor viability after passaging, 10 μM ROCK inhibitor Y-27632 may be included in the medium during the plating step.
Increasing Workflow Flexibility: NPC Cryopreservation and Thawing
Banking your NPCs at a midpoint in your experimental protocol offers flexibility and allows you to standardize your downstream experiments by starting with the same initial NPC population. After neural induction, NPCs may be cryopreserved using either STEMdiff™ Neural Progenitor Freezing Medium or CryoStor® CS10 following the instructions outlined in section 6.3 of the Technical Manual (Document #10000005588).
Important considerations for NPC thawing:
- If thawing for immediate use in a downstream application:
- Cryopreserved NPCs should be thawed into STEMdiff™ NPM. Allow the cells to recover for one day prior to changing to the desired downstream medium.
- Seeding density upon thawing may need to be optimized to yield the ideal density for the application.
- If thawing for subsequent NPC expansion:
- Cryopreserved NPCs should be thawed into STEMdiff™ NPM and maintained until they are ready to be passaged (typically 5 - 7 days).
- Suggested seeding density upon thawing is 200,000 live cells/cm2.
- Recharacterize neural marker expression (PAX6, SOX1, Nestin) after thawing.
- Avoid repeated freezing and thawing of the same batch of cells. Try to bank NPCs at relatively early passage numbers and use these stocks for experiments.
- Post-thaw viability is typically 70 - 85%. If you observe poor NPC recovery after plating, 10 μM ROCK inhibitor Y-27632 may be included in the medium during the plating step.
Resources for hPSC-Based Neural Induction and Differentiation
Browse scientific posters, webinars, and technical tips for culturing neural cells from hPSCs.
Protocol Considerations for Downstream Differentiation to Neurons and Astrocytes
If you plan to further differentiate your cells into neurons or astrocytes, there are multiple culture workflow options available. Please refer to the following table for details:
Day 12 - 13 of the embryoid body neural induction protocol or immediately following the 18- to 21-day monolayer neural induction protocol, as outlined in the STEMdiff™ Forebrain Neuron Product Information Sheet (Document #10000005464).
Note: It is not recommended to initiate the midbrain neuron differentiation following the full 17- to 19-day embryoid body neural induction.
*The alternative workflow options listed above are compatible with NPCs derived using the STEMdiff™ Neural Induction System, but reduced downstream differentiation efficiency may be observed compared to the recommended workflow.
Still unsure which workflow is right for your research? We can help. Contact us by phone or email or use the LiveChat function on this page to discuss your specific needs and applications.
References
- Meyers EA et al. (2017) TGF-β Family Signaling in Neural and Neuronal Differentiation, Development, and Function. Cold Spring Harb Perspect Biol 9(8):a022244.
- Chambers SM et al. (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling, Nat Biotech 27:275—80.
Related Neural Induction & Differentiation Products
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration