Healthy Control Human iPSC Line, Female, SCTi003-A
Human pluripotent stem cell line, frozen

Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
Overview
To ensure optimal product performance and reproducibility, SCTi003-A is manufactured using extensive quality control procedures in a culture system consisting of mTeSR™ Plus, Corning® Matrigel® hESC-Qualified Matrix, and ReLeSR™. SCTi003-A is karyotypically stable, demonstrates trilineage differentiation potential, expresses undifferentiated cell markers, and was reprogrammed using a non-integrating reprogramming technology. Registration with hPSCreg® ensures ethical and biological conformity based on community standards.
Validated for expansion in the PBS-MINI Bioreactor, the SCTi003-A cell line provides a pathway for efficient scale-up of your cultures. It has also been validated with STEMdiff™ kits across multiple lineages and tissue types in both 2D and organoid models (Figures 6-11). Browse TeSR™ and STEMdiff™ cell culture media products to establish a complete workflow for your cell culture system.
This research-use-only (RUO) product has been consented for both academic and commercial use. Blood samples are ethically sourced using Institutional Review Board (IRB) or other regulatory authority-approved consent forms and protocols. For donor details and cell quality characterization of the source cell banks refer to the data figures on this page. SCTi003-A is derived from an αβ T cell and has undergone VDJ sequence rearrangement. For additional details, refer to the lot-specific Certificate of Analysis and Frequently Asked Questions About Induced Pluripotent Stem Cell Lines.
Whole exome and whole genome sequence data files are available upon request. Please contact us for pricing.
Certain products are only available in select territories. Please contact your local sales representative or Product & Scientific Support at techsupport@stemcell.com for further information.
Data Figures
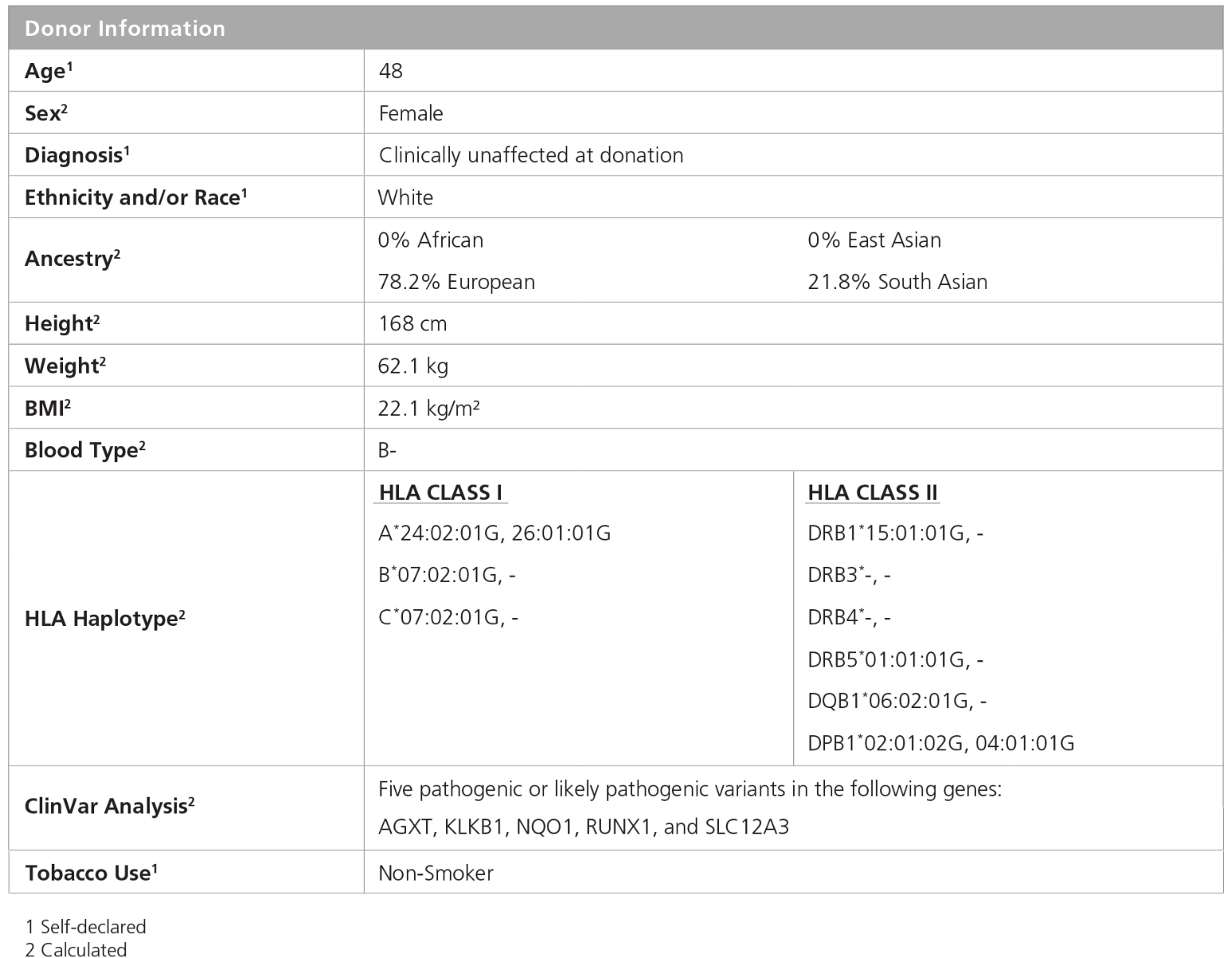
Figure 1. iPSC Line SCTi003-A Is Derived from a Healthy Female Donor
Demographic, health, and genetic characteristics of the SCTi003-A donor are compiled based on self-reported information and whole-exome sequencing. Sex was determined by karyotype. Ancestry was calculated by EthSEQ analysis from whole-exome sequencing data. HLA haplotype was determined by next-generation sequencing, sequence-base typing, and sequence-specific oligonucleotide probes as needed to obtain the required resolution. Other genetic variants were determined from whole-exome sequencing using ClinVar analysis. Blood type (ABO/Rh blood group) was determined by next-generation sequencing. Height, weight, and BMI were calculated at the donation facility. iPSC = induced pluripotent stem cell.
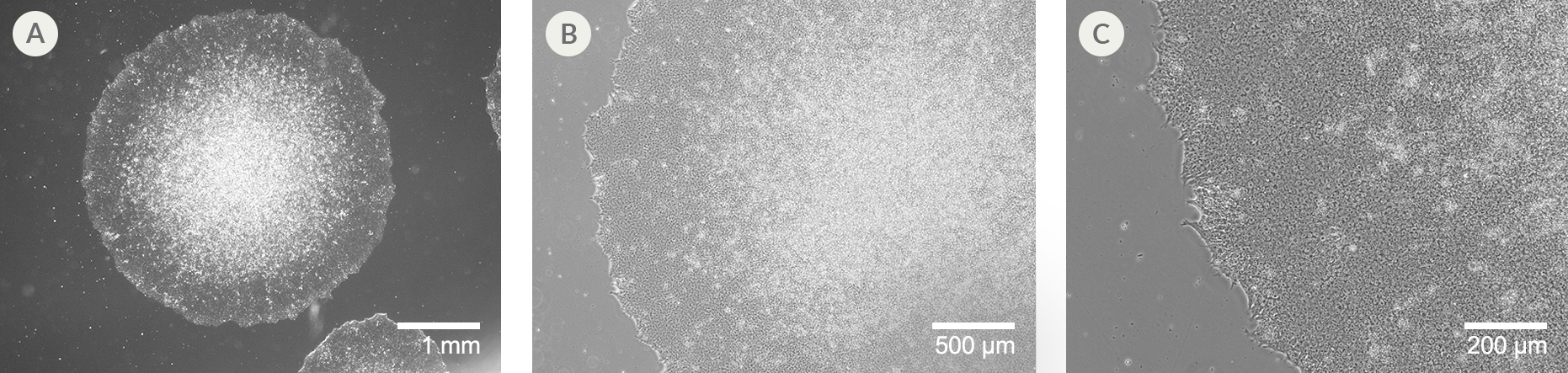
Figure 2. SCTi003-A Human Pluripotent Stem Cells Demonstrate High-Quality Morphology in Routine Culture
Cryopreserved cells from line SCTi003-A were thawed and maintained in mTeSR™ Plus on Corning® Matrigel® Matrix. (A) The resulting iPSC colonies have densely packed cells and show multi-layering when ready to be passaged. (B,C) Cells retain prominent nucleoli and high nuclear-to-cytoplasmic ratios. iPSC = induced pluripotent stem cell.
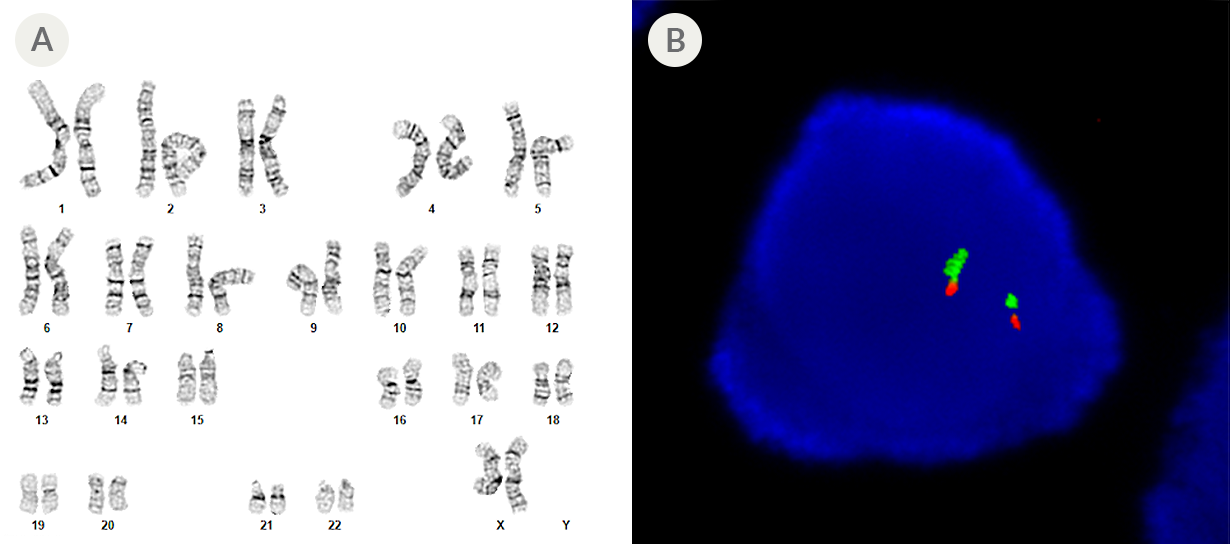
Figure 3. SCTi003-A Human Pluripotent Stem Cells Maintain a Normal Karyotype
(A) G-T-L banding for thawed cells at p26 (n = 20) shows a normal karyotype with no evidence of clonal abnormalities at a band resolution of 450 - 550 G-bands per haploid genome. (B) Fluorescent in situ hybridization in a representative p26 iPSC using probes for 20p11 (green) and 20q11.21 (red). 94% of cells examined displayed two sets of two probe signals, indicating no aneusomy of chromosome 20 (n = 200). iPSC = induced pluripotent stem cell.
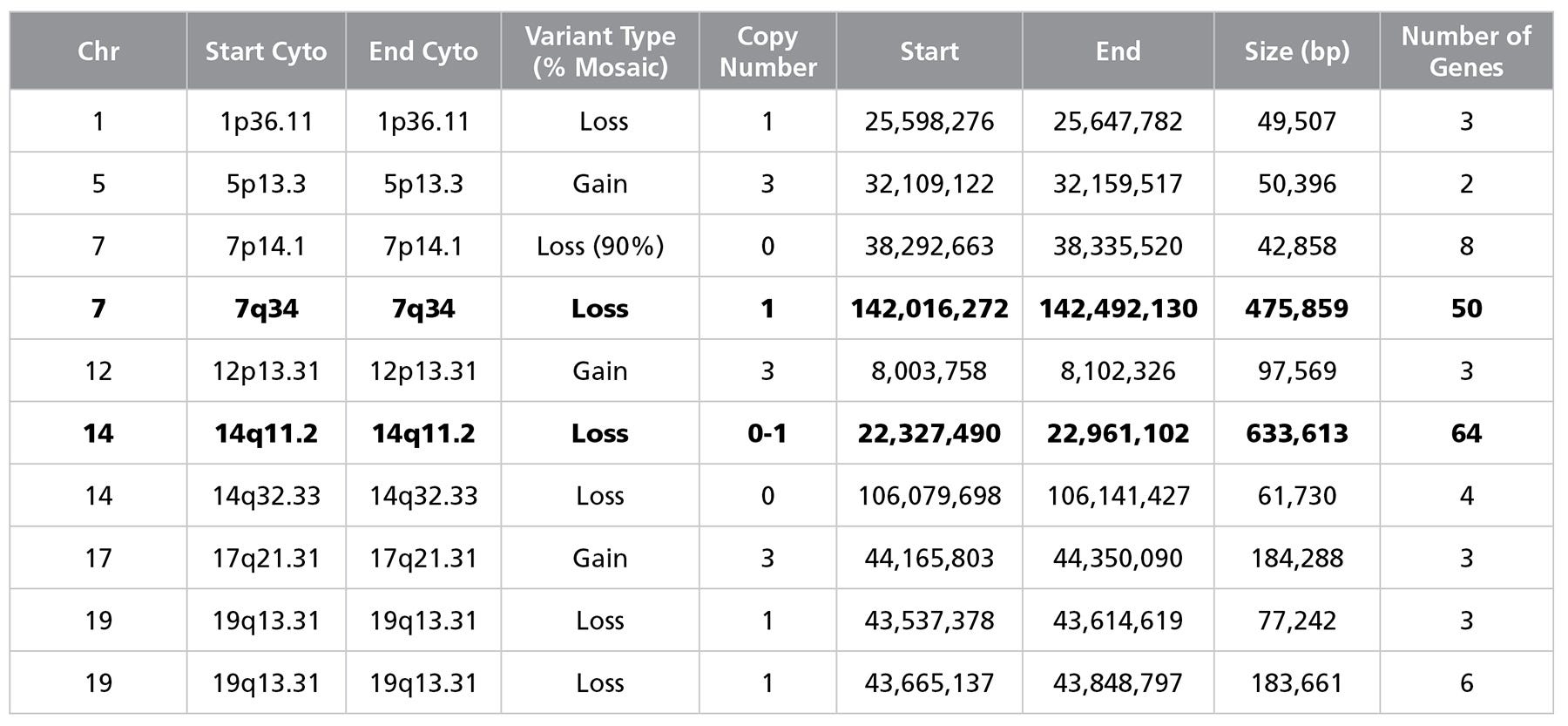
Figure 4. Single Nucleotide Polymorphism Microarray Analysis Characterizes SCTi003-A Copy Number Variants
DNA was extracted from a vial of SCTi003-A from the Master Cell Bank and subject to SNP microarray analysis to identify large-scale copy number variants (CNVs). The cells display two reportable CNVs, defined as those greater than 400kb in size, on chromosome 7 and 14 (rows highlighted in bold font). These losses are located in the TCR regions of the genome and are indicative of VDJ recombination process during T Cell development. Array design, genomics position, genes, and chromosome banding are based on genome build GRCh37/hg19. chr = chromosome; start cyto = cytogenetic band at the start of the base pair imbalance; end cyto = cytogenetic band at the end of the base pair imbalance; bp = base pairs; SNP = single nucleotide polymorphism; TCR = T Cell Receptor; VDJ = variable, diversity, joining segment.
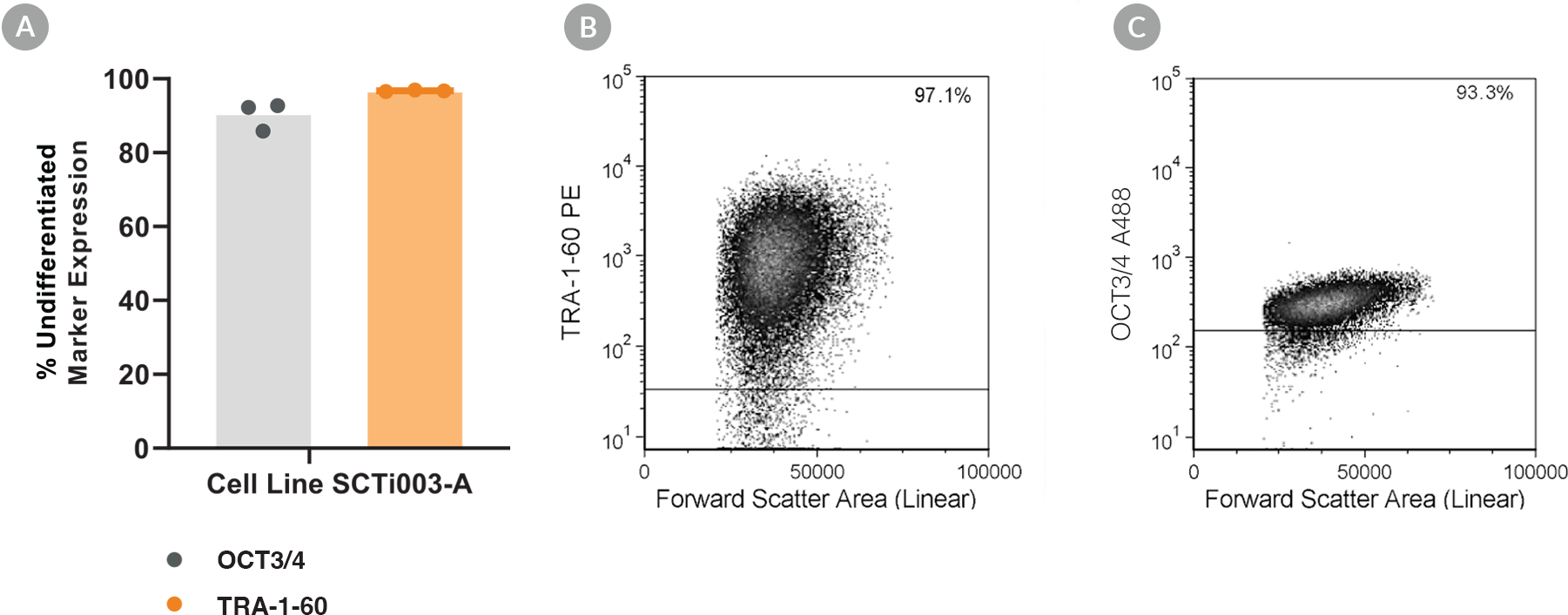
Figure 5. SCTi003-A iPSCs Express Undifferentiated Cell Markers
Cell line SCTi003-A was characterized using flow cytometry for undifferentiated cell markers OCT3/4 and TRA-1-60. (A) Percentage marker expression was quantified 5 passages after thawing from the Master Cell Bank from analyses of three technical replicates. Representative flow cytometry plots are displayed for (B) TRA-1-60 and (C) OCT3/4. iPSC = induced pluripotent stem cell.
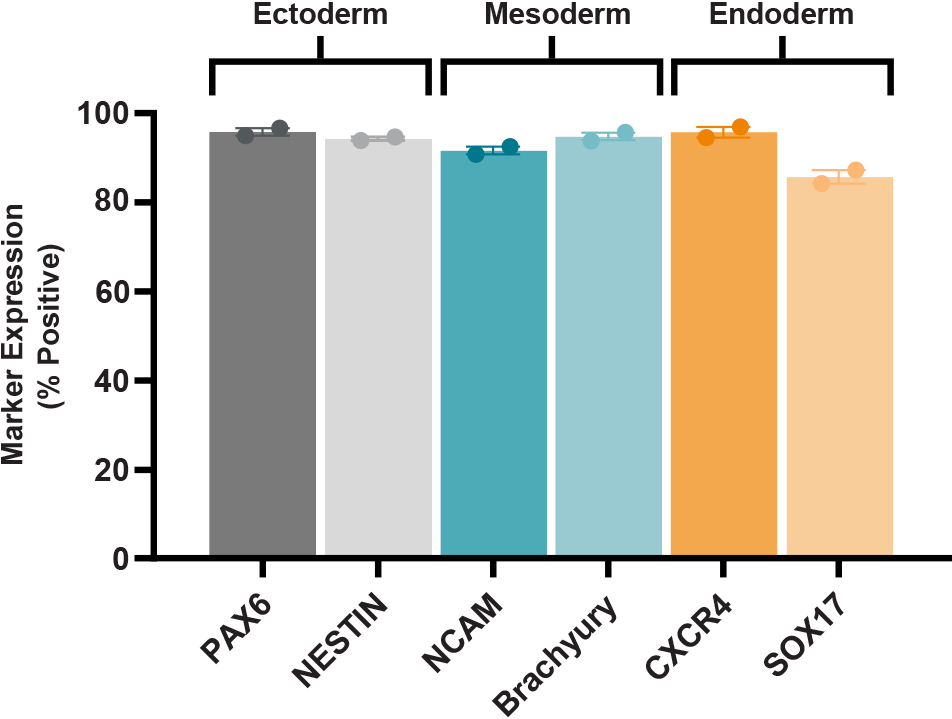
Figure 6. SCTi003-A Human Pluripotent Stem Cells Demonstrate a High Trilineage Differentiation Capacity
Cells from SCTi003-A were split into 3 groups, differentiated using STEMdiff™ Trilineage Differentiation Kit (#05230), and then subjected to flow cytometry analysis. Two markers for each embryonic germ layer were assessed, and bars present mean marker expression for each group of cells (n = 2 biological replicates). PAX6 and Nestin confirm differentiation to the ectoderm lineage, NCAM and Brachyury (T) to the mesoderm lineage, and CXCR4 and SOX17 to the endoderm lineage.
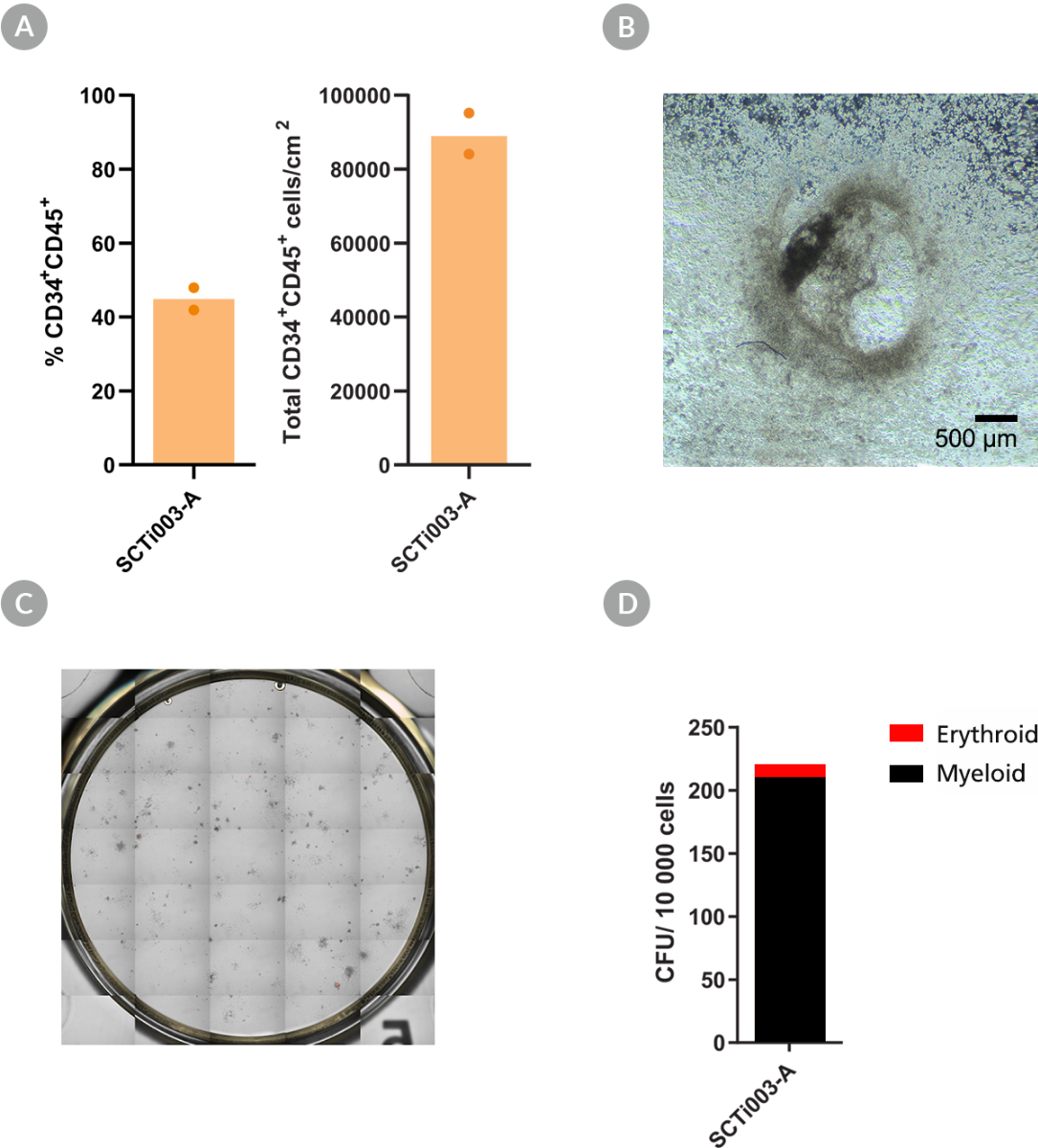
Figure 7. SCTi003-A iPSCs Can Form Hematopoietic Progenitor Cells and Have Colony-Forming Potential
STEMdiff™ Hematopoietic Kit (#05310) was used to generate HPCs from the SCTi003-A cell line. (A) Percentages and yields of CD34+CD45+ HPCs per cm2 after differentiation (n = 2 biological replicates). (B) Brightfield image of SCTi003-A-derived HPCs indicates that these cells transitioned through a typical endothelial-to-hematopoietic transition (EHT). The resulting HPCs were then subject to a CFU assay (n = 2) with MethoCult™ SF H4636. (C) After 14 days of incubation, colonies were imaged with STEMvision™ and (D) enumerated from digital images. Both myeloid (black) and erythroid (red) colonies can be formed from this line. iPSCs = induced pluripotent stem cells; HPCs = hematopoietic progenitor cells; CFU = colony-forming unit.
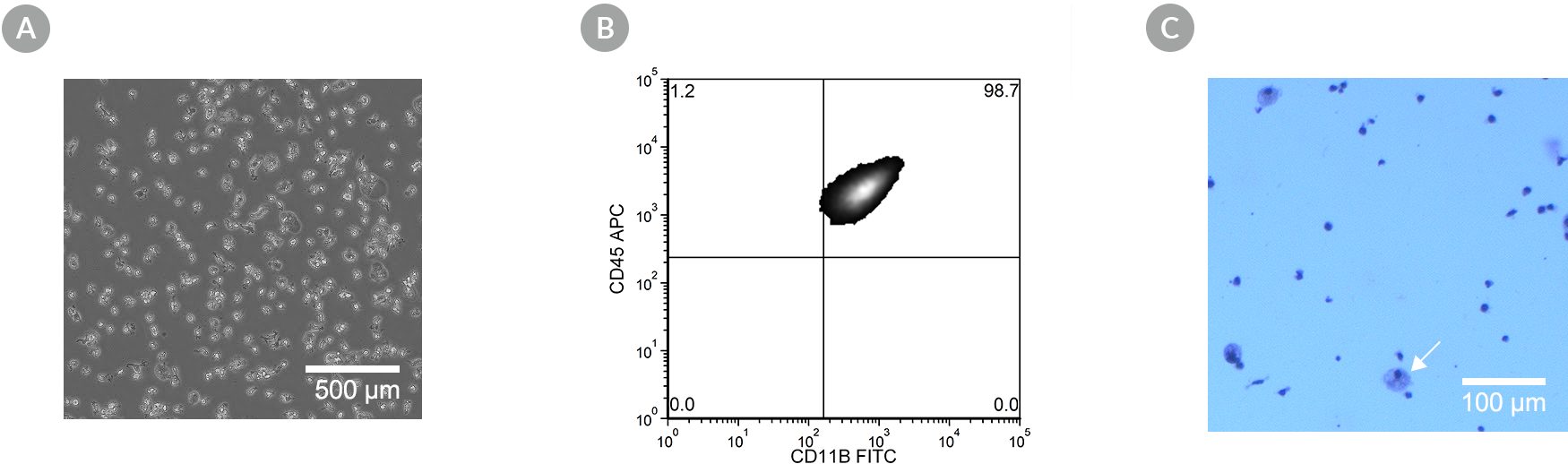
Figure 8. SCTi003-A Human Pluripotent Stem Cells Can Effectively Differentiate into Microglia
Hematopoietic progenitor cells generated from cell line SCTi003-A using the STEMdiff™ Hematopoietic Kit (#05310) were further differentiated using STEMdiff™ Microglia Differentiation and Maturation Kits (#100-0019, #100-0020). (A) The resulting cells are small with visible processes, are non-adherent on Matrigel®, and exhibit small cytoplasmic-to-nuclear ratios characteristic of microglia. (B) Co-expression of CD45 and CD11b was observed by flow cytometry. (C) The resulting cells are also adherent on poly-D-lysine, and contain < 20% monocyte-like cells (large, with lightly stained cytoplasm; arrow) as assessed by May-Grunwald Giemsa stain at Day 27.
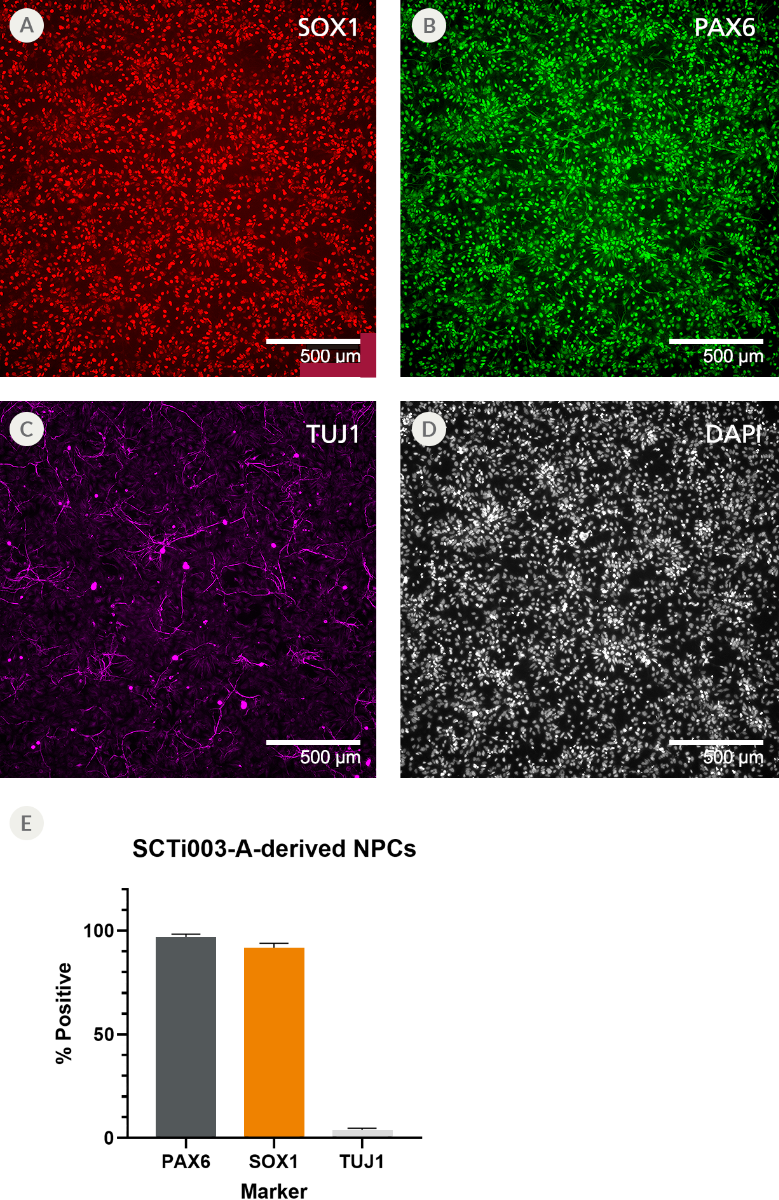
Figure 9. SCTi003-A Human Pluripotent Stem Cells Can Efficiently Differentiate into Neural Progenitor Cells
NPCs were generated from SCTi003-A iPSCs using STEMdiff™ SMADi Neural Induction Kit (#08581) following the monolayer protocol in the Product Information Sheet, and subsequently cryopreserved. The resulting NPCs were thawed, established in culture, and fixed for immunocytochemistry. The NPCs express neural progenitor markers (A) SOX1 and (B) PAX6 with low expression of (C) class III β-tubulin (TUJ1). (D) In addition, they display the expected small, teardrop-shaped morphology. (E) Marker expression was quantified and found to be greater than 90% for neural progenitor markers and less than 10% for mature neuronal markers. Error bars represent standard deviation (n = 2 biological replicates). NPCs = neural progenitor cells; iPSCs = induced pluripotent stem cells.
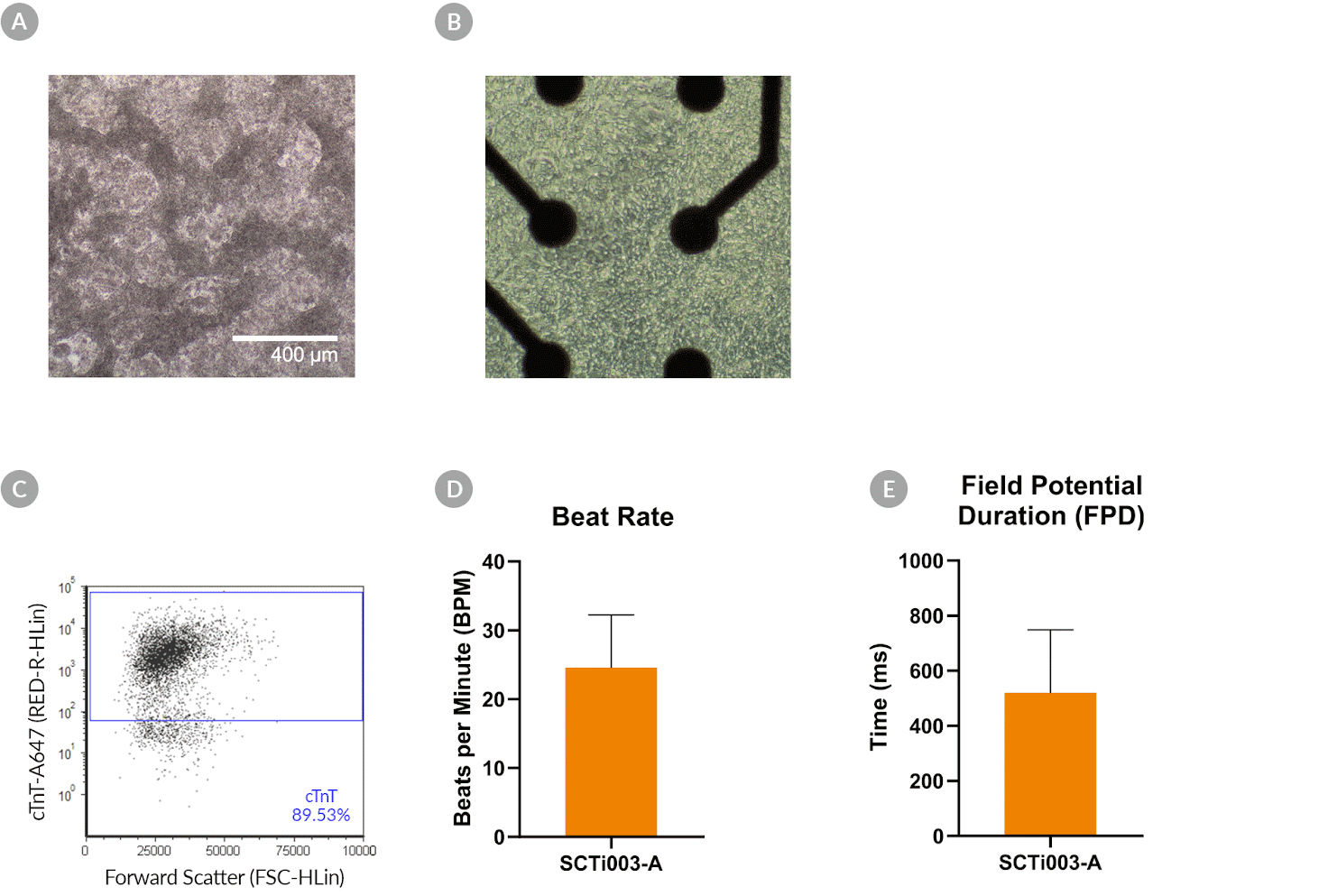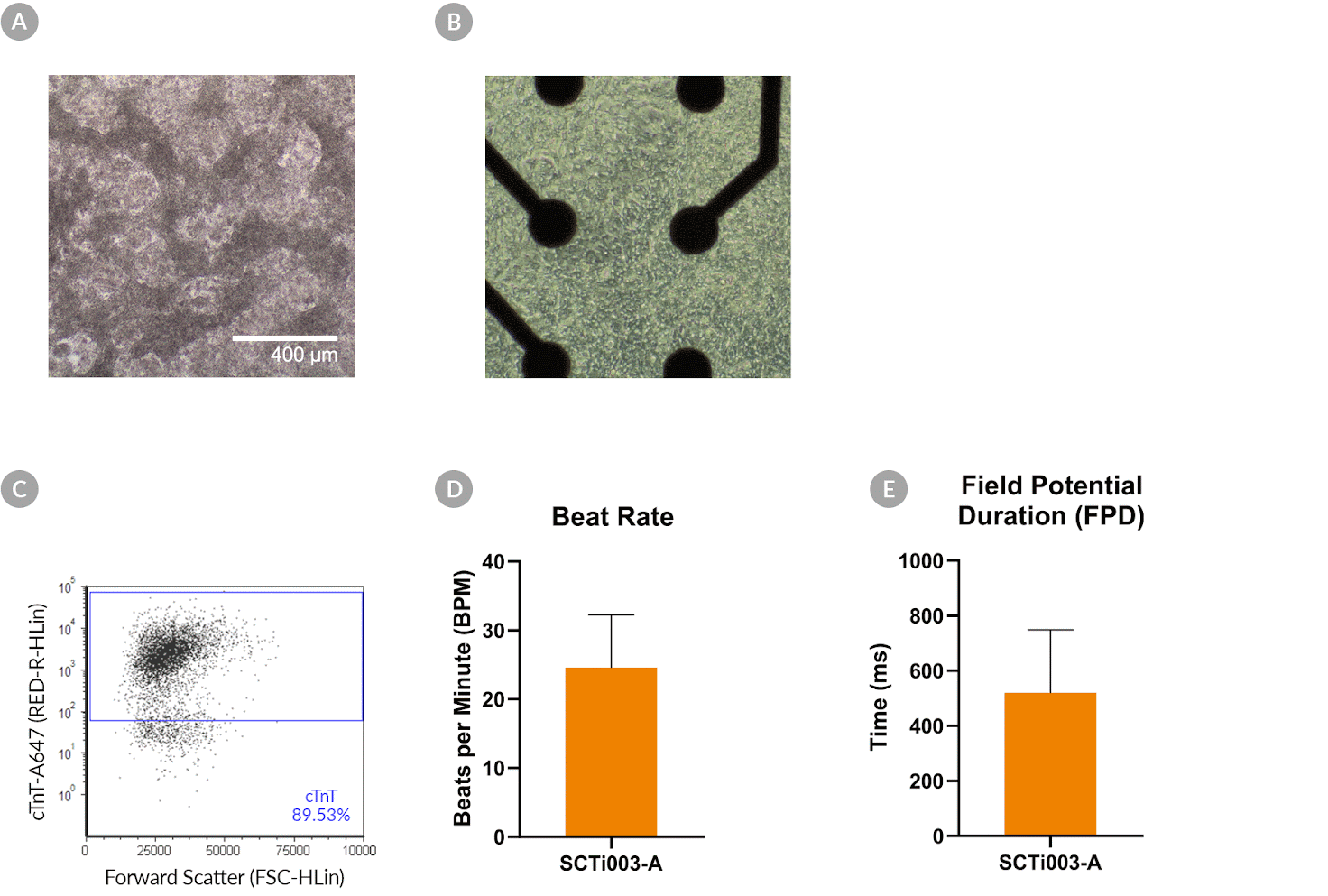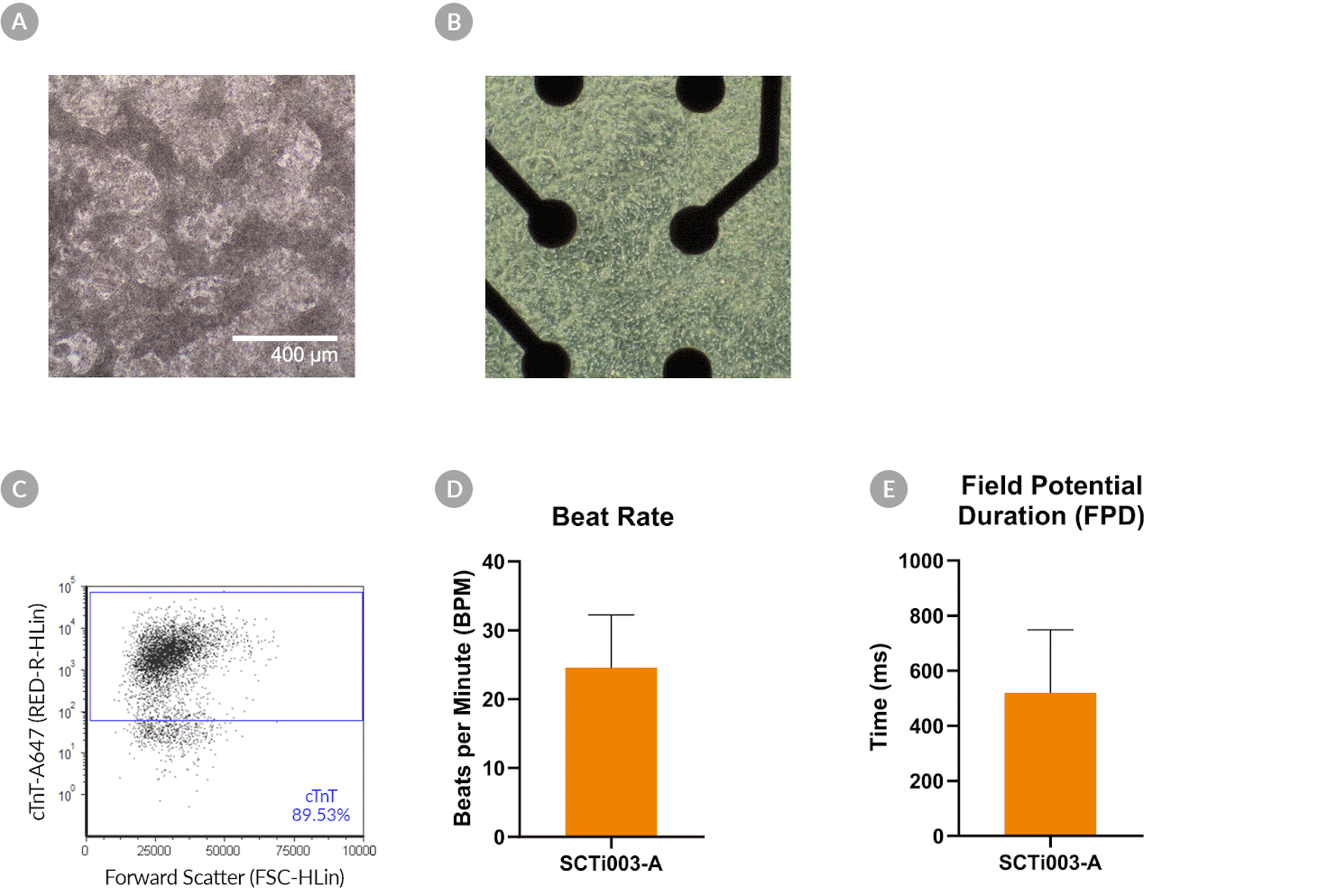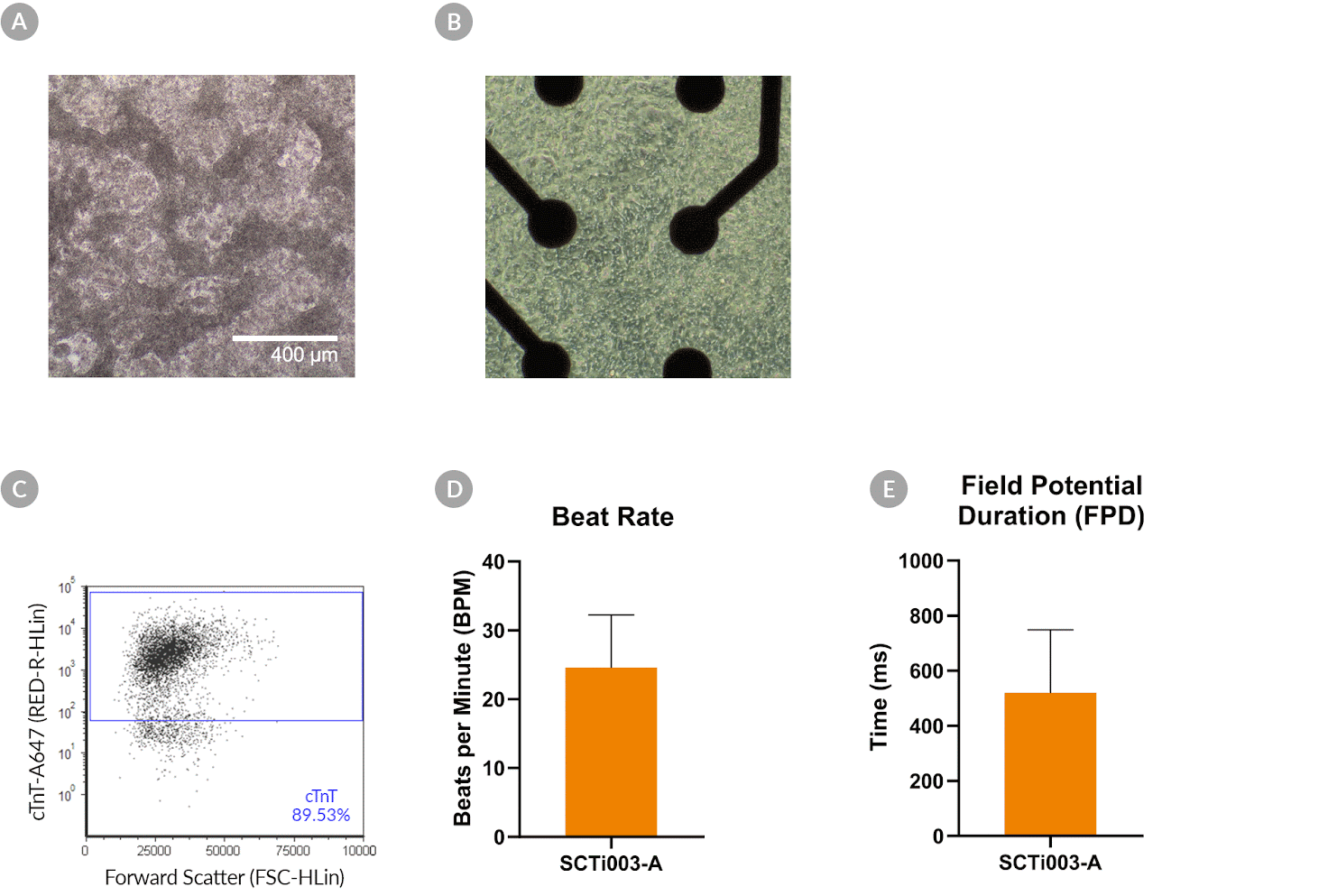
Figure 10. SCTi003-A Human Pluripotent Stem Cells Can Successfully Differentiate into Ventricular Cardiomyocytes
Ventricular cardiomyocytes were generated from SCTi003-A iPSCs using STEMdiff™ Ventricular Cardiomyocyte Differentiation Kit (#05010). (A) Monolayer cultures at Day 15 of differentiation show iPSC-derived ventricular cardiomyocytes that exhibit beating behavior. (B) Beating ventricular cardiomyocytes can be replated and maintained in a well of an MEA plate for functional analysis. (C) Of the resulting cells, 89% express cardiomyocyte marker cTnT, as detected by flow cytometry. (D) The iPSC-derived ventricular cardiomyocytes at Day 22 of differentiation beat at ~25 BPM (n = 3 replicates, mean +/-SD plotted) and (E) have a field potential duration of ~500 ms (n = 3 replicates, mean +/-SD plotted), as assessed by MEA. iPSCs = induced pluripotent stem cells; MEA = microelectrode array.
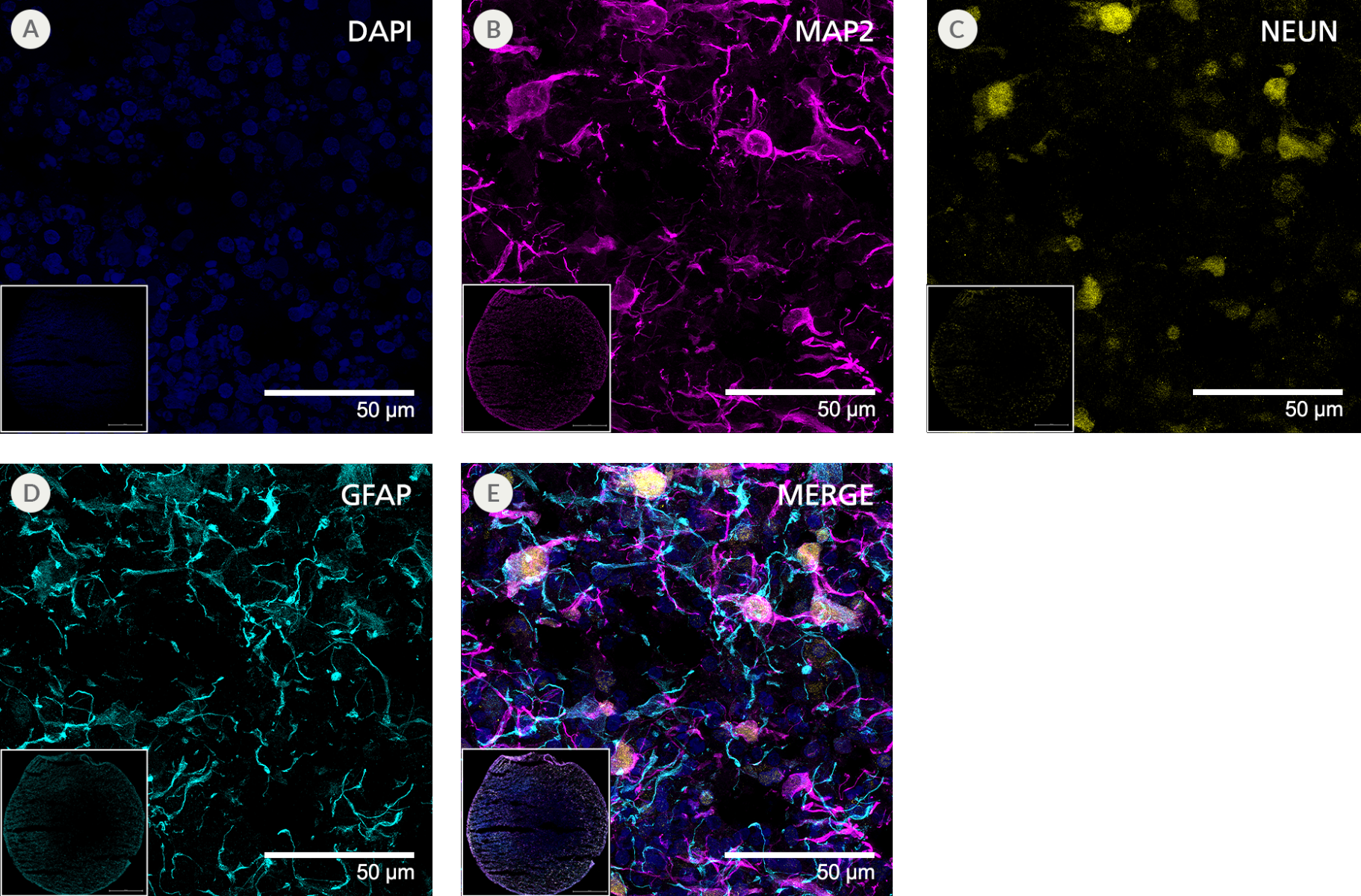
Figure 11. SCTi003-A Human Pluripotent Stem Cells Can Successfully Differentiate into Neural Organoids
iPSCs from SCTi003-A were differentiated to neural organoids using STEMdiff™ Dorsal Forebrain Organoid Differentiation Kit (#08620). Dorsal forebrain organoids were maintained until Day 105 with STEMdiff™ Neural Organoid Maintenance Kit (#100-0120) before fixing, cryosectioning, and immunofluorescent staining. The resulting organoids were stained for (A) DAPI (blue), (B) MAP2 (magenta), (C) NEUN (yellow), and (D) GFAP (cyan). (E) 4-channel merged image. Panels show cellular-level detail at 63x magnification. Insets show the full cryosection at 10x magnification. iPSCs = induced pluripotent stem cells.
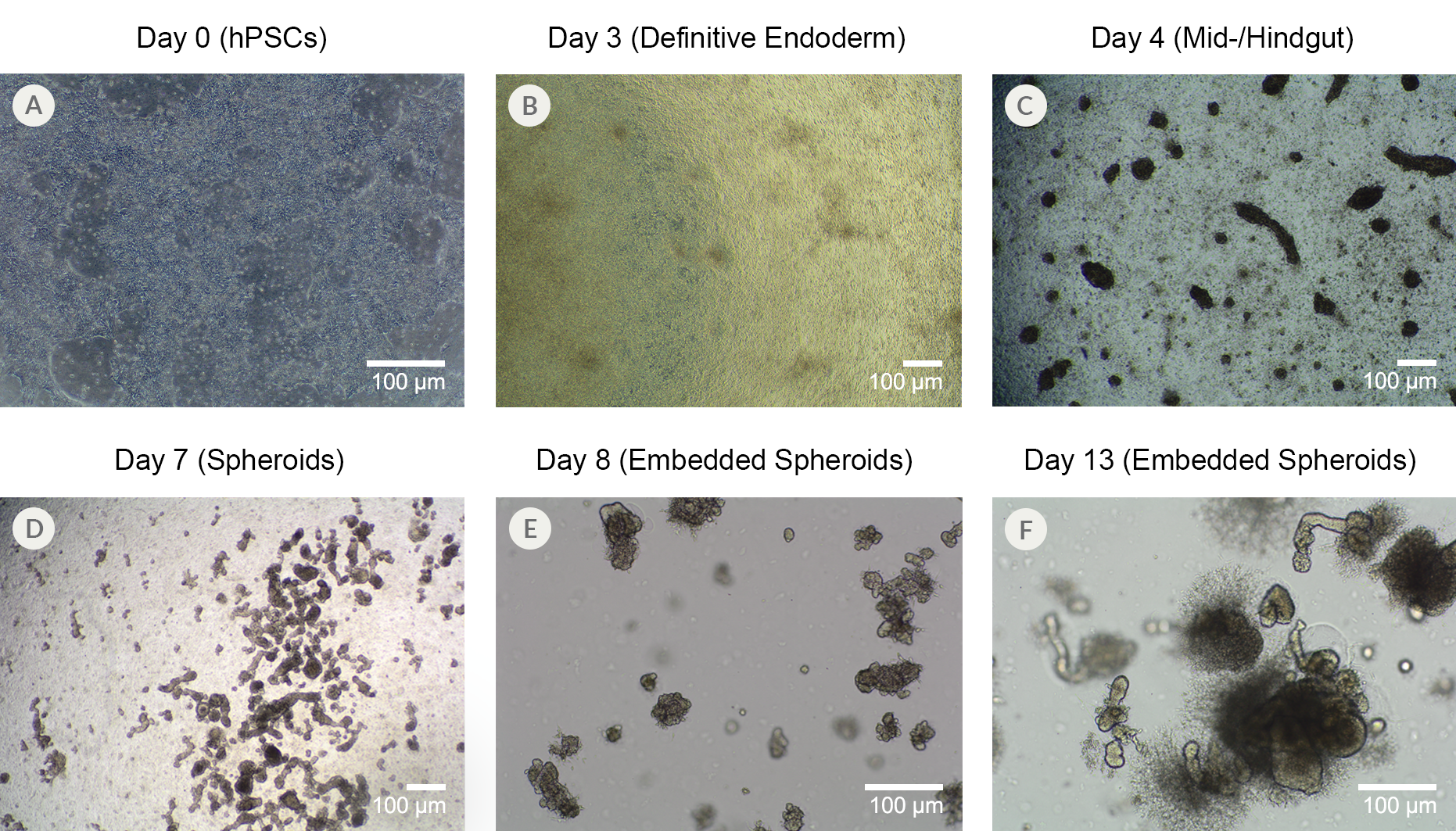
Figure 12. Human Pluripotent Stem Cells from Line SCTi003-A Can Successfully Differentiate into Intestinal Organoids
(A) SCTi003-A iPSCs were plated down for use with STEMdiff™ Intestinal Organoid Kit (#05140). (B) By Day 3 of the protocol outlined in the Product Information Sheet, the monolayers show more uniformity and display characteristics of patterning to definitive endoderm. (C) After switching to mid-/hindgut medium, 3D structures become visible atop the monolayer culture, and (D) spheroids detach from the mid-/hindgut culture at Day 7 of differentiation. (E) Collections of spheroids can be embedded in Matrigel® domes for subsequent maturation into human intestinal organoids, which (F) expand significantly after just 6 days in matrix. The maturing organoids can be passaged and expanded using STEMdiff™ Intestinal Organoid Growth Medium (#05145). iPSCs = induced pluripotent stem cells.
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Resources and Publications
Educational Materials (38)
Related Products
-
 ReLeSR™
ReLeSR™cGMP, enzyme-free human pluripotent stem cell selection and passaging reagent
-
 Vitronectin XF™
Vitronectin XF™Defined, xeno-free matrix that supports the growth and differentiation of human pluripotent stem cells under serum-free, feeder-free conditions
-
 mTeSR™ Plus
mTeSR™ PluscGMP, stabilized feeder-free maintenance medium for human ES and iPS cells
-
 ThawSTAR® CFT2 Automated Thawing System
ThawSTAR® CFT2 Automated Thawing SystemAutomated cell thawing system for consistent thawing performance
-
 CloneR™2
CloneR™2Defined supplement for improving survival of human ES and iPS cells in single-cell workflows
Item added to your cart

Healthy Control Human iPSC Line, Female, SCTi003-A
Legal Statement:
These iPSCs and their modifications (including but not limited to derivatives or differentiated progeny) shall not be used or administered in (1) human subjects for human clinical use; (2) animals for veterinary use for therapeutic, diagnostic, or prophylactic purposes or (3) any subject in relation to clinical applications, cell therapy, transplantation, and/or regenerative medicines, without limiting the generality of the foregoing.
These iPSCs and their modifications (including but not limited to derivatives or differentiated progeny) may not be used for monetization or commercialization purposes, including without limitation, used to, or with the
goal to, perform services or supply products or rights, including in the manufacture of cellular therapies or other therapeutics, for monetary gain or the generation of royalties, revenues, sales or other valuable
consideration. For clarity, these iPSCs and their modifications (including but not limited to derivatives or differentiated progeny) may not be used for screening compounds, antibodies, proteins or peptides, except
for the purposes of target discovery, target validation, or assay development, provided such activities and the results of such activities are not further used for monetization or commercialization purposes.
It may be possible to obtain a further license for the prohibited uses referred to in this Limited Use License. Please contact iPSCrequests@stemcell.com for more details.
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED.
Copyright © 2022 by STEMCELL Technologies Inc. All rights reserved including graphics and images. STEMCELL Technologies & Design, STEMCELL Shield Design, Scientists Helping Scientists, CloneR, ReLeSR,
and STEMdiff are trademarks of STEMCELL Technologies Canada Inc. mTeSR and TeSR are trademarks of WARF. Corning, Falcon, and Matrigel are registered trademarks of Corning Incorporated. CryoStor and
ThawStar are registered trademarks of BioLife Solutions. All other trademarks are the property of their respective holders. While STEMCELL has made all reasonable efforts to ensure that the information provided by
STEMCELL and its suppliers is correct, it makes no warranties or representations as to the accuracy or completeness of such information.
Quality Statement:
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.






































