Neural Induction of hPSCs Using the Embryoid Body Method
STEMdiff™ Neural Induction Medium is a defined, serum-free medium for the efficient & reproducible derivation of neural progenitor cells
Neural induction is the process by which human pluripotent stem cells (hPSCs), including human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells, are specified to a neuroectodermal fate. Neural induction may be achieved through either an embryoid body (EB) or monolayer method. For details to help you choose the best workflow for your neural induction, please see Designing Your Neural Induction and Differentiation Workflow.
In the sections below, we highlight key protocol tips and important considerations to keep in mind when performing EB-based neural induction using STEMdiff™ Neural Induction Medium (Catalog #05835) or STEMdiff™ SMADi Neural Induction Kit (Catalog #08581). The full protocol may be found in the Technical Manual for Generation and Culture of Neural Progenitor Cells Using the STEMdiff™ Neural System (Document #10000005588).
If you plan to use the monolayer option instead, please see Neural Induction of hPSCs Using the Monolayer Method.
hPSC Quality for Embryoid Body Neural Induction
It is critical to use high-quality hPSCs to ensure efficient neural induction. This means that no more than 5 - 10% of the colonies in the hPSC culture display morphological indications of spontaneous differentiation. Any areas that appear differentiated must be marked and removed prior to initiating neural induction. Visit our hPSC Cell Quality Center to learn more about how you can assess and maintain high-quality hPSC cultures.
Key Protocol Steps & Tips for Embryoid Body Neural Induction
- Pre-warm medium before use. Do not add cold medium from the refrigerator directly to your cells.
- Day 0: The AggreWell™800 (Catalog #34811) plate must be pre-treated with Anti-Adherence Rinsing Solution (Catalog #07010) prior to seeding cells. Failure to do so will result in suboptimal EB formation.
- Day 0: Ensure that 10 μM Y-27632 (ROCK inhibitor, Catalog #72302) is included in the seeding medium to promote good survival of single-cell hPSCs.
- Day 1-4: Medium changes during the AggreWell™ stage must be done very slowly and carefully, so as not to disturb the EBs from the microwells. When removing or adding medium, keep the edge of the pipette tip against the side of the well, just below the surface of the medium.
- Day 1-5: EBs should be visible by Day 1, and should appear round and symmetrical, with smooth, defined borders. Asymmetrical growth or failure of cells to incorporate into a defined EB may indicate poor hPSC quality. If EBs do not display good morphology during this stage, it is recommended to restart the protocol.
- Day 1-5: It is normal for the medium to appear yellow by the time of the daily feed, as long as the medium is not turbid and the EBs display good morphology.
- Day 5: After replating the EBs, ensure that they are evenly distributed throughout the well by shaking the plate back and forth, side to side. Do not swirl the plate or move it in a figure-8 motion. This can promote EB clumping, potentially leading to difficulty with scoring on Day 8 and inefficient rosette selection on Day 12.
- Day 8: Calculating the % neural induction by scoring rosettes is an important checkpoint. The neural induction score should be at least 75%.
- Day 12: The purpose of the neural rosette selection step is to collect the neural rosettes that contain central nervous system neural progenitor cells (NPCs) while leaving behind the flat cells that have grown out from the rosettes (the majority of which are likely to be neural crest cells).
- Day 12: Do not overselect (e.g. do not perform excessive washing or over-incubation with the STEMdiff™ Neural Rosette Selection Reagent). It is better to underselect (leaving some rosettes behind) and sacrifice yield than to overselect (taking along the flat cells) and sacrifice purity.
- Day 12: Try not to break up the rosettes too much when collecting them. Some breaking up is acceptable, but avoid excessive trituration.
- Day 13 - Day 17-19: Sometimes the cultures can appear very confluent soon after rosette replating. As long as the cells are not dying or lifting off the plate, it is preferable to continue the protocol as written until at least Day 17, rather than to passage early.
- Day 17-19: Even if the neural induction score on Day 8 was high, the resulting NPCs still need to be validated at the end of the induction by assessing neural marker expression (PAX6, SOX1, Nestin). When performing the first passage, seed a subset of cells into a smaller well format for immunocytochemistry analysis. For more information, please see Designing Your Neural Induction and Differentiation Workflow.
- Day 17-19: When replating cells into STEMdiff™ Neural Progenitor Medium (Catalog #05833) after the first passage, ensure that cells are seeded at the recommended density (e.g. at least 1.25 x 105 cells/cm2). NPCs can pack together very closely and may appear confluent soon after passaging. However, culturing the cells at a high density is important for maintaining their proliferative, progenitor state.
Representative Culture Morphology for Embryoid Body Neural Induction
Refer to the following representative culture images for a general idea of what may be observed at various stages of the neural induction EB protocol using the STEMdiff™ SMADi Neural Induction Kit.
Important Note
Although NPC morphology can provide information about culture health, it is not a reliable method for confirming neural induction and purity. To assess the efficiency of neural induction, immunocytochemistry is recommended. For detailed information on this as well as tips for further maintenance of your NPCs, please see Designing Your Neural Induction and Differentiation Workflow.
Users may see some variation in their own cultures compared to these images. For example, different cell lines may form rosettes more or less efficiently, may be distributed more or less homogeneously, and/or may proliferate more quickly or slowly. Deviation from the morphology depicted below does not necessarily mean the experiment will fail.
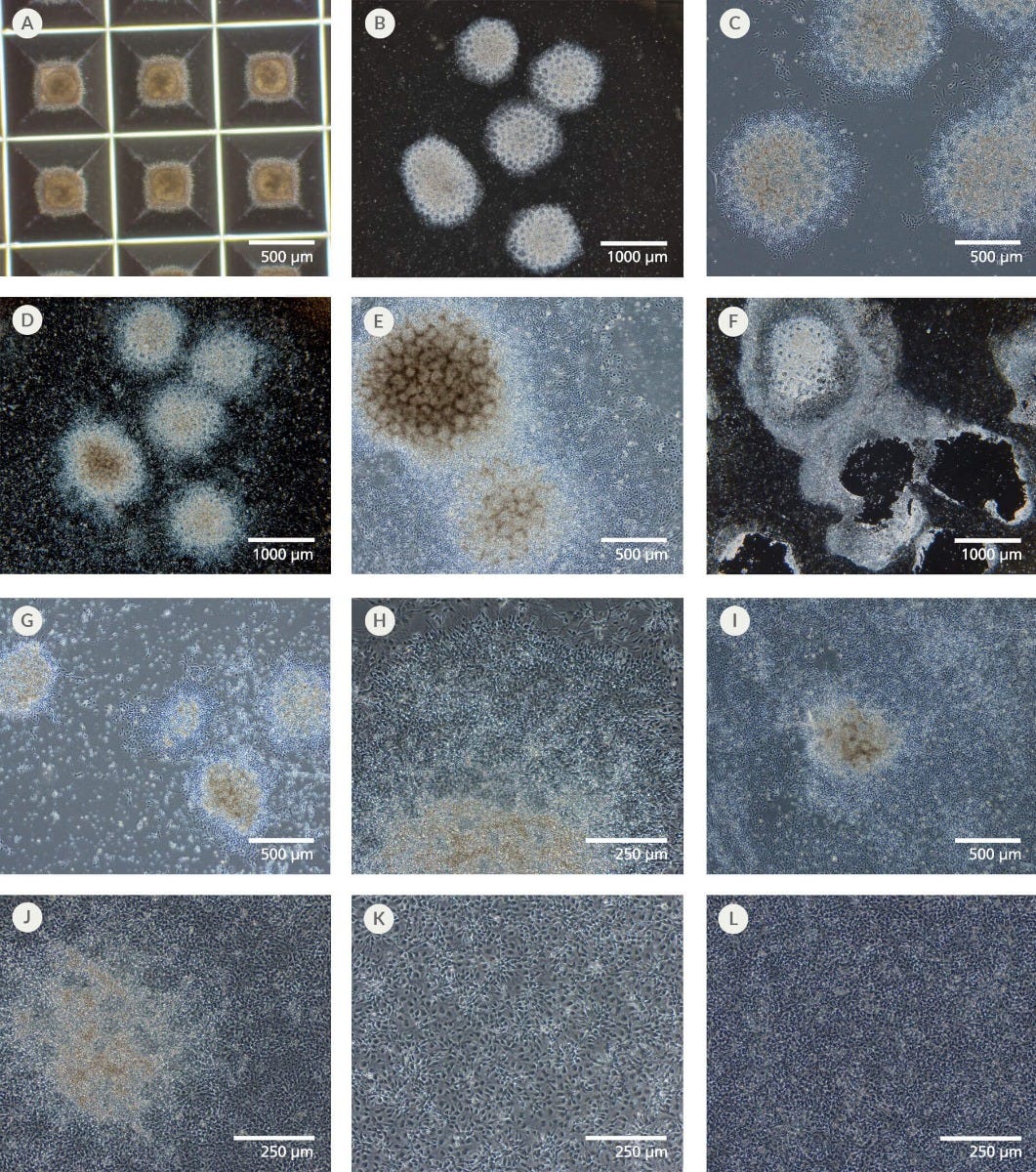
Figure 1. Representative Culture Morphology of Embryoid Body Neural Induction
(A) Day 5 EBs in an AggreWell™800 plate (4X objective). EBs appear round and symmetrical, with smooth edges. (B) Day 8 neural rosettes (2X objective). All EBs in this field of view exhibit > 50% neural rosette structures and would be counted as positive EBs in the neural induction scoring step. (C) Day 8 neural rosettes (4X objective). Outgrowth of flat cells from the edges of the rosettes may be observed. (D) Day 12 neural rosettes before selection (2X objective). (E) Day 12 neural rosettes before selection (4X objective). (F) Day 12 original plate after neural rosette collection (2X objective). Most of the neural rosettes have been collected, leaving behind the flat cells. A few rosettes may be left behind. (G) Day 13 replated neural rosettes (4X objective). The replated rosette clusters have adhered back down to the new plate and NPCs are beginning to grow out from them. (H) Day 15 neural rosettes (10X objective). A densely packed monolayer of NPCs can be observed growing out farther from the neural rosette cluster. (I) Day 17 - 19 neural rosettes (4X objective). By the time the cultures are ready to be passaged, the NPCs have proliferated to cover most of the spaces between the replated rosette clusters. (J) Day 17 - 19 neural rosettes (10X objective). By the time the cultures are ready to be passaged, the NPCs form a tightly packed monolayer in the spaces surrounding the neural rosettes. Secondary rosette patterns in the monolayer may or may not be visible. (K) NPCs 1 day post-passage (10X objective). Passaging has broken up the rosettes and outgrowing NPCs, allowing them to be replated as a dense, homogeneous monolayer. NPCs appear partially elongated with teardrop-shaped cell bodies and minimal neurite outgrowth. (L) NPCs 6 - 7 days post-passage (10X objective). NPCs have proliferated and packed together very tightly, such that it is difficult to distinguish individual cells. The cultures are ready for the next passage.
Frequently Asked Questions About Embryoid Body Neural Induction
What is the proper nomenclature for NPCs throughout passaging? What is considered p0, p1, etc?
Are there any points in the EB neural induction protocol when it is possible to double feed and/or skip feeds?
What should be done if the neural rosettes are not detaching during the selection step on Day 12?
Important: It is better to underselect than to overselect at this step. It is preferable to leave behind a few firmly-attached rosettes than to carry forward the contaminating flat cells surrounding them.
Is a wide-bore pipette tip required to resuspend and transfer the neural rosettes on Day 12?
Should I passage early if the cultures appear confluent very soon after the neural rosette replating step on Day 12?
How many NPCs can be expected at the end of the EB neural induction?
Still have questions about the EB neural induction protocol? We can help. Contact us by phone or email or use the LiveChat function on this page to discuss your specific needs and applications.
Related Resources
On-Demand Neural Induction Course
Learn about the basic principles of neural induction and choosing the workflow that’s right for you, all at your own pace with this comprehensive digital course.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration