BrainPhys™ Neuronal Media for Optimal Neuronal Functionality
BrainPhys™ Neuronal Culture Media
Where Neuronal Activity Meets Scientific Potential
Accurate disease modeling and predictive drug screening rely on neuronal cultures that closely replicate the physiological environment of the brain. Whether you are leveraging human pluripotent stem cell (hPSC)-derived neurons to study disease mechanisms or using rodent neuronal models for preclinical research, the reliability and relevance of your results depend on your ability to reproducibly create a physiologically relevant brain-like environment.
Neurons must be active to be functional, yet traditional culture media prioritize neuronal survival over neuronal activity. For more physiologically relevant research, it is essential to support both neuronal activity and maturity in culture. Optimized neuronal media can help achieve this balance.
Promote, rather than inhibit, neuronal activity and maturity in your cultured neurons with BrainPhys™ media. Read on to discover how the BrainPhys™ family of neuronal media offers a range of optimized solutions for neural research, allowing you to choose the best fit for any stage of your neural culture workflow. Additionally, explore key resources to streamline and optimize your neural workflows.
Why Use BrainPhys™?
- Choose a culture medium that mimics the extracellular environment of the brain.
- Improve neuronal function and increase the proportion of synaptically active neurons.
- Conduct functional assays without the need to perform medium changes.
- Support the long-term culture of primary or hPSC-derived neurons.
- Achieve minimal lot-to-lot culture variability due to rigorous raw material screening and quality control.
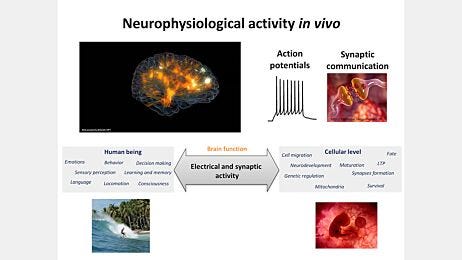
Why Neuronal Activity Matters
Cultures with functional neuronal activity mimic the complexity of the brain by demonstrating key processes such as synaptic transmission, plasticity, and neural network formation. These features play a crucial role in applications such as drug screening, disease modeling, and regenerative medicine. Neurons must be active to be functional—failing to simulate physiological conditions can lead to impaired neuronal function, reduced synaptic activity, developmental immaturity, and unreliable experimental results. BrainPhys™ media provide the physiologically relevant environment needed to sustain neuronal activity, ensuring more accurate and reliable research outcomes in these applications.
Cells that fire together wire together
When an axon of cell A is near enough to excite a cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A's efficiency, as one of the cells firing B, is increased.
Hebb, D.O. (1949). The Organization of Behavior. New York: Wiley & Sons.
How BrainPhys™ Compares to Other Neuronal Culture Media
Traditional media used for culturing primary and hPSC-derived neurons were designed to support survival rather than promote neuronal function. These media often have low osmolarity, nonphysiological salt and glucose concentrations, and may contain neuroactive components that inhibit synaptic activity. These conditions lead to impairment of critical functions such as action potential generation and synaptic transmission.
To temporarily address these limitations, researchers typically transition cultures into artificial cerebrospinal fluid (ACSF) for data recording. However, frequent medium changes can stress neurons, and prolonged exposure to nonphysiological media before recording remains a challenge. Since neuronal activity directly influences cell signaling, survival, morphology, gene expression, and subcellular protein/organelle localization, maintaining neurons in physiological conditions throughout maturation creates a model that is stable and better reflects the in vivo central nervous system.
BrainPhys™ provides a more physiological environment that promotes, rather than inhibits, neuronal activity and maturity. Explore the figures below to see how BrainPhys™ compares to other neuronal culture media for culturing various neuronal models, including primary and hPSC-derived neurons.

Figure 1. BrainPhys™ Neuronal Medium with NeuroCult™ SM1 Neuronal Supplement Supports Higher Neural Activity Than Other Commercially Available Culture Systems
Primary E18 rat cortical neurons were cultured with glucose-supplemented* BrainPhys™ and NeuroCult SM1 Neuronal Supplement or other commercially available culture systems with its recommended supplement for 8 weeks. Neuronal activity can be detected by multi-electrode array (MEA) at Day 9 with BrainPhys™, whereas activity is not detected until Day 14 in cultures maintained in either of the other commercially available media with its recommended supplement. In the Commercial Medium condition, a widely used commercially available neuronal medium, the mean firing rate remains low throughout the culture period. In contrast, a “peak-drop” activity pattern is observed in the Commercial Medium “Plus” condition, where the mean firing rate increases rapidly within 2 days, followed by a drop in activity in the next 2 - 4 days. BrainPhys™and SM1 Kit with 15 mM glucose maintains the highest level of activity throughout the 8-week culture period.
*For applications that require particularly high seeding densities, such as MEA that results in greater-than-usual energetic demand, supplementation of BrainPhys™ with additional glucose (12.5 - 17.5 mM, e.g. final concentration of 15 - 20 mM) may be beneficial. We recommend optimizing for the lowest concentration that provides you with success in your application. The timing of supplementation should also be optimized. Cultures may be supplemented as soon as a culture is transitioned into BrainPhys™, but beginning the supplementation after 1-2 weeks in BrainPhys™ may also yield acceptable results.

Figure 2. hPSC-Derived Neurons Matured in BrainPhys™ Neuronal Medium Show Improved Excitatory and Inhibitory Synaptic Activity
Neural progenitor cells (NPCs) were generated from H9 cells using STEMdiff™ Neural Induction Medium in an embryoid body-based protocol. Next, NPCs were cultured for 44 days in vitro in (A,C) BrainPhys™ Neuronal Medium, supplemented with 2% NeuroCult™ SM1 Supplement, 1% N2 Supplement-A, 20 ng/mL GDNF, 20 ng/mL BDNF, 1 mM db-cAMP, and 200 nM ascorbic acid to initiate neuronal differentiation, or (B,D) in DMEM/F-12 under the same supplementation conditions. (A,C) Neurons matured in BrainPhys™ Neuronal Medium showed spontaneous excitatory (AMPA-mediated; A) and inhibitory (GABA-mediated; C) synaptic events by voltage-clamp electrophysiology. The frequency and amplitude of spontaneous synaptic events are consistently greater in neuronal cultures matured in BrainPhys™ Neuronal Medium compared to neurons plated and matured in DMEM/F-12 (B,D). Traces are representative.
How to Incorporate BrainPhys™ into Your Cultures
Whether you use commercial media-based protocols or take a do-it-yourself (DIY) approach for your neuronal cultures, BrainPhys™ seamlessly integrates into your culture workflow. You can easily transition from other commercial media to BrainPhys™ for more functional cell cultures and complete your workflow with BrainPhys™-containing STEMdiff™ maturation kits. Alternatively, BrainPhys™ can be tailored to your specific needs for a customized approach—whether for differentiation, maturation, long-term characterization assays, or all three. Figure 3 below highlights key stages where BrainPhys™ can be used to improve the maturation and function of your 2D cultures.

Figure 3. BrainPhys™ Integrates Seamlessly into 2D Neural Workflows for Various Neuronal Cell Types
BrainPhys™ can be incorporated at key stages in hPSC and rodent neuronal culture workflows. For hPSC-derived neuronal models, standardized STEMdiff™ culture medium kits for generating four neuronal cell types (motor, sensory, forebrain, and midbrain) rely on BrainPhys™-based maturation, while the two glial subtypes that can be generated by STEMdiff™ kits (astrocytes and microglia) are compatible with BrainPhys™-based co-culture systems. Alternatively, hPSC-derived neurons may be generated using a DIY protocol incorporating BrainPhys™, as illustrated above. Additionally, BrainPhys™ supports downstream applications, including long-term culture and characterization assays. For primary rodent model systems, neuron cultures can be established using the standardized BrainPhys™ Primary Neuron Kit or a DIY protocol incorporating BrainPhys™, followed by BrainPhys™ for downstream applications.
BrainPhys™ Neuronal Medium is well-suited for short-term 3D neuronal applications, such as electrical recordings of neural organoids (Figure 4), and can also be incorporated for long-term neural organoid maturation. Additional supplementation may be required and should be optimized as needed.

Figure 4. STEMdiff™ Spinal Cord Organoids Display Increased Electrophysiological Activity When Matured in BrainPhys™
BrainPhys™ was incorporated into a standardized spinal cord organoid workflow (Catalog #100-1524) by transitioning organoids from STEMdiff™ Spinal Cord Differentiation Medium to BrainPhys™ supplemented with STEMdiff™ Neural Organoid Supplement A, with medium changes every 2 - 3 days. Spinal cord organoids matured in BrainPhys™ Neuronal Medium + STEMdiff™ Neural Organoid Supplement A displayed higher electrophysiological activity as measured by MEA compared to those matured in STEMdiff™ Neural Organoid Maintenance Kit Medium (Catalog #100-0120), with increased spikes, active electrodes, weighted mean firing rate (WMFR), burst number and frequency, and synchrony index. Data were normally distributed (D’Agostino & Pearson test) and analyzed with a paired t-test (n = 3 cell lines, 1 - 3 technical replicates; * p ≤ 0.05,** p ≤ 0.01).
Which BrainPhys™ Medium Is Right for Your Research?
Your research questions are specific, so we've developed specialized formulations of BrainPhys™ to meet your diverse research needs. Whether your focus is on live fluorescent imaging, enhanced synaptic activity, long-term culture stability, or specific assay compatibility, there's a medium designed for your application. Explore the unique benefits of each to find the perfect fit for your research.
Resources to Advance Your 2D Workflows
Neuronal Cell Culture
How to Tri-Culture hPSC-Derived Forebrain Neurons, Astrocytes, and Microglia
Produce a functionally active model of neuronal-glial interactions and neuroinflammation with this optimized 2D co-culture protocol, supported by BrainPhys™.
How to Co-Culture hPSC-Derived Forebrain Neurons and Microglia
Use our step-by-step guides for co-culturing hPSC-derived microglia and forebrain neurons to model neuroimmune interactions, supported by BrainPhys™.
Assessing Functional Activity
Culturing hPSC-Derived Forebrain Neurons for MEA Analysis Using the Maestro MEA™ System
Explore this step-by-step protocol for culturing hPSC-derived forebrain neurons on CytoView MEA™ plates to produce mature, consistent, and stable neuronal activity using Maestro MEA™ systems.
Culturing Primary Rodent Neurons for MEA Analysis Using the Maestro MEA™ System
Follow this step-by-step protocol to culture embryonic Day 18 (E18) rat cortical neurons using BrainPhys™ Neuronal Medium on CytoView MEA™ plates for measurable, consistent, and stable neuronal activity using Maestro MEA™ systems.
Measuring Activity in hPSC-Derived Astrocytes Generated with the STEMdiff™ Astrocyte SF Culture System
View this poster to learn how to generate highly purified, functional astrocytes under serum-free conditions, with calcium signaling assessment supported by BrainPhys™ Imaging Optimized Medium.
Resources to Advance Your 3D Workflows
Assessing Functional Activity
Measuring Activity in hPSC-Derived Guided Neural Organoids
Watch this webinar to discover standardized workflows for generating midbrain, cerebral, and spinal cord organoids and methods to measure functional outputs of these neural organoids using MEAs for drug discovery and disease modeling.
Measuring Unguided Neural Organoid Activity on 3D High-Density MEAs
Explore the recording of neuronal activity from organoids on a multi-electrode array surface, where the 3D pillar structure of the array enhances tissue-electrode coupling in large 3D tissues.
Generating hPSC-Derived Spinal Cord Organoids and Assessing Their Functional Output
View this poster to learn how to efficiently generate functional spinal cord organoids containing motor neurons, interneurons, and glial cells using STEMdiff™ Spinal Cord Organoid Differentiation Kit, and see MEA data showcasing their functional output.
Other Tools for Your Workflow
STEMdiff™ hPSC Differentiation Product Explorer
Find the right STEMdiff™ kit for your hPSC differentiation, with our interactive product explorer. Select an organ system to browse the cell types and organoids you can generate with our standardized STEMdiff™ medium kits.
Antibodies Targeting Neural Markers
Successfully evaluate hPSC-derived neuronal differentiation with our new STEMdiff™ Neural System-compatible antibodies that target MAP2, SOX2, PAX6, and Synaptophysin.
Cytokines and Recombinant Proteins
Ensure effortless integration into your neural experiments with cytokines and recombinant proteins verified for optimal performance in relevant workflows, including BrainPhys™ and NeuroCult™.