IntestiCult™ Organoid Differentiation Medium (Human)
Cell culture medium for further differentiation of human intestinal organoids in 3D, or as monolayers/air-liquid interface cultures
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
-
 IntestiCult™ Organoid Growth Medium (Human)
IntestiCult™ Organoid Growth Medium (Human)Cell culture medium for establishment and maintenance of human intestinal organoids
-
 Gentle Cell Dissociation Reagent
Gentle Cell Dissociation ReagentcGMP, enzyme-free cell dissociation reagent
-
 Costar® 6.5 mm Transwell®, 0.4 µm Pore Pol...
Costar® 6.5 mm Transwell®, 0.4 µm Pore Pol...Polystyrene plate with lid and polyester membrane inserts for cell culture that feed basolaterally
-
 Y-27632 (Dihydrochloride)
Y-27632 (Dihydrochloride)RHO/ROCK pathway inhibitor; Inhibits ROCK1 and ROCK2
-
Labeling Antibodies
Compatible antibodies for purity assessment of isolated cells
What Our Scientist Says
Organoids have truly expanded the limits of what's possible for in vitro studies of the intestinal epithelium. By providing optimized culture media and robust, approachable protocols, we are making these technologies more accessible to researchers.

Overview
Intestinal cultures generated using IntestiCult™ Organoid Differentiation Medium (Human) contain physiologically relevant proportions of differentiated and stem cell populations, recapitulating the diversity of the crypt-villus axis. When compared to conventional cell lines, intestinal monolayers exhibit greater barrier integrity, express higher levels of key differentiation markers, and have a morphology that is more representative of the in vivo intestine.
Applications of intestinal organoid cultures include studying the development and function of the intestinal epithelium, modeling intestinal diseases, compound screening, and regenerative therapy approaches. Intestinal monolayer and ALI cultures are particularly amenable for permeability assays and studies of infectious diseases due to easy access to the apical surface. This kit requires IntestiCult™ Organoid Growth Medium (Human; Catalog #06010) for the initiation and expansion of intestinal organoids prior to differentiation.
Learn how to culture human intestinal organoids in our On-Demand Intestinal Course or browse our Frequently Asked Questions (FAQs) about the organoid workflows using IntestiCult™. Additionally, download our detailed e-book Proven Protocols for Intestinal Organoid Culture: Getting Started with IntestiCult™ for a curated collection of intestinal organoid protocols.
Should you intend to use this product for commercial purposes, please contact HUB Organoids B.V. at www.huborganoids.nl for a commercial use license or for clarifications in relation to HUB Organoids B.V. licensing.
More Information
| Safety Statement | CA WARNING: This product can expose you to Progesterone which is known to the State of California to cause cancer. For more information go to www.P65Warnings.ca.gov |
|---|
Data Figures
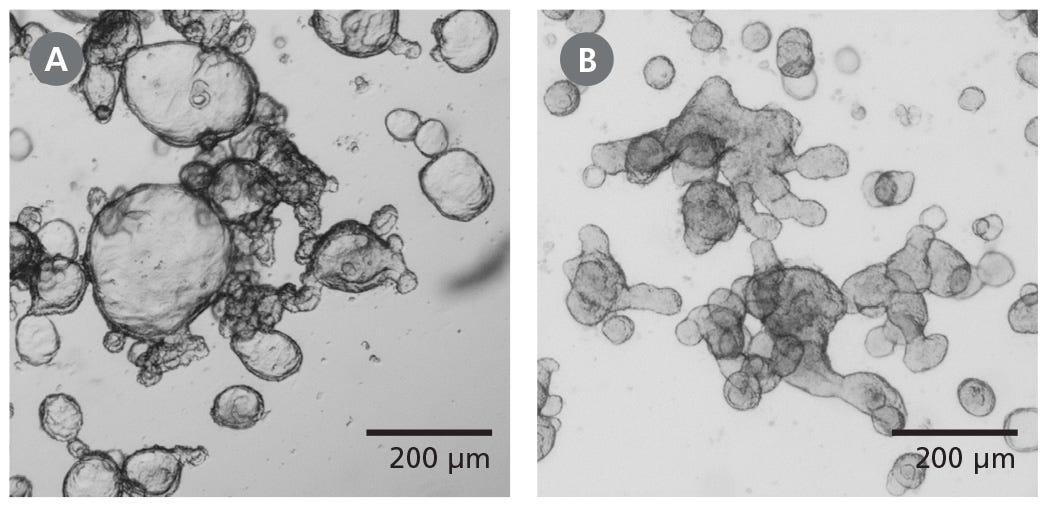
Figure 1. Differentiated Human Intestinal Organoids Display a Budded Morphology
(A) Organoids grown in IntestiCult™ OGM are primarily cystic. (B) When switched to IntestiCult™ ODM, organoids develop a thickened epithelium with a pronounced, budded morphology indicative of a more differentiated state. Organoids were imaged on day 5 of expansion or differentiation respectively.
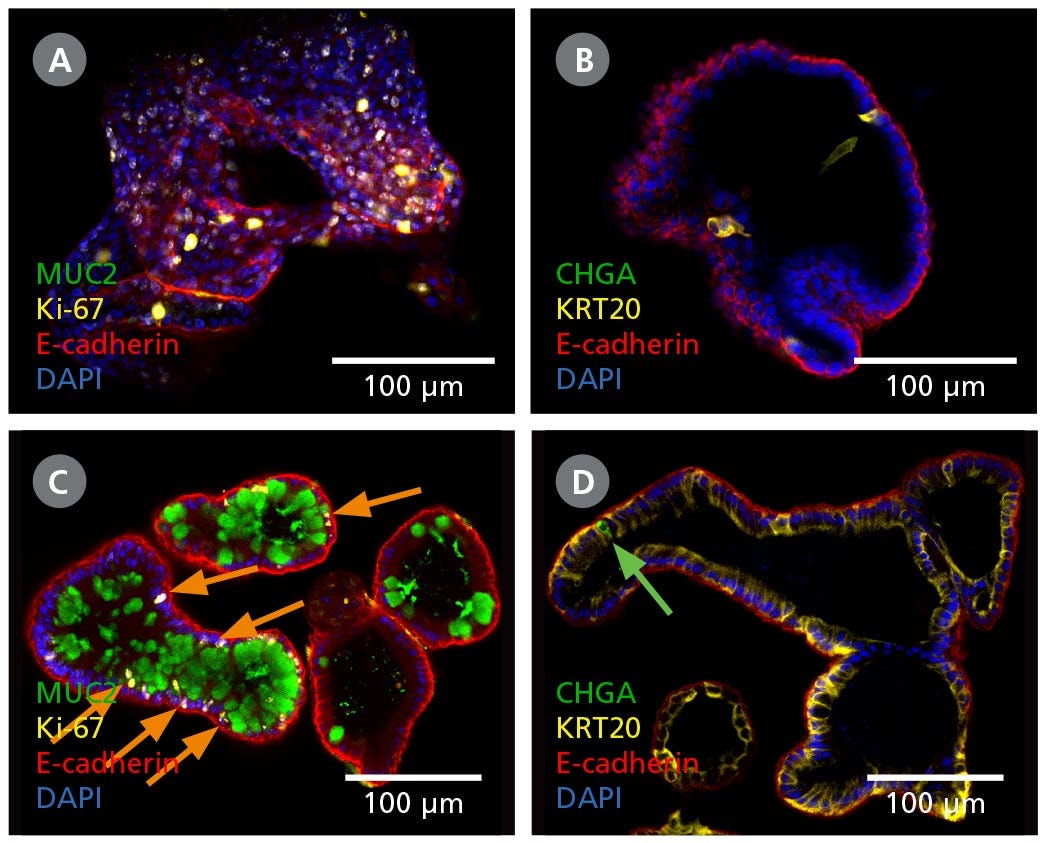
Figure 2. Intestinal Organoids Contain a Higher Proportion of Mature Cell Types Following Differentiation in IntestiCult™ ODM
(A, B) Organoids grown in IntestiCult™ OGM are enriched for Ki-67+ proliferative cells (A), while containing few differentiated cell types such as goblet cells (MUC2, A), enterocytes (KRT20, B), and enteroendocrine cells (CHGA, B). (C, D) When switched to IntestiCult™ ODM, organoids contain a small number of Ki-67+ proliferative cells (C, arrows), with more physiological proportions of goblet cells (MUC2, C), enterocytes (KRT20, D), and chromogranin A- (CHGA-)positive enteroendocrine cells (D, arrow).
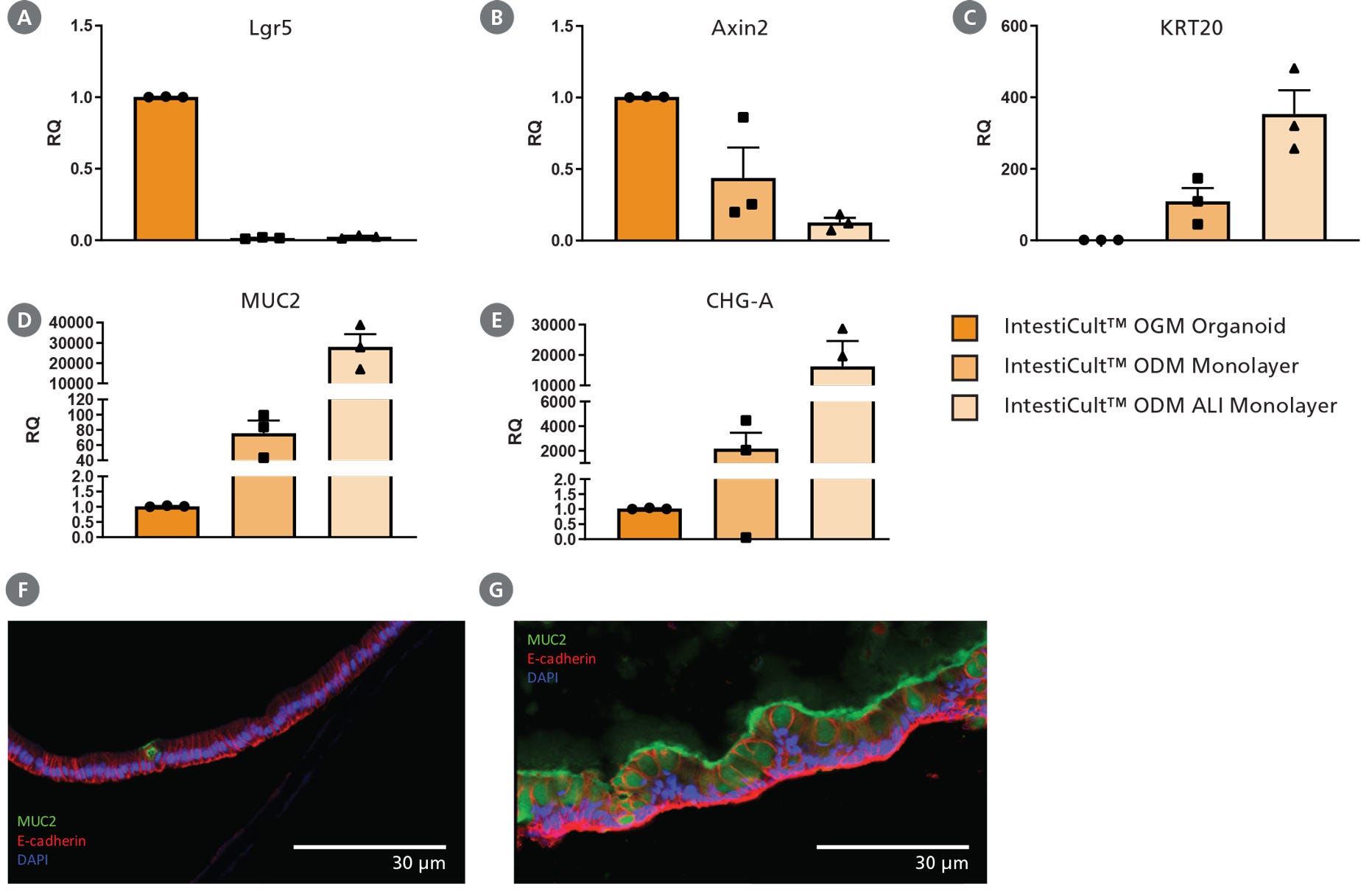
Figure 3. Differentiation of Intestinal Epithelium at the Air-Liquid Interface (ALI) Using IntestiCult™ ODM
(A – E) Growing organoid-derived monolayers as ALI cultures drives further differentiation of intestinal epithelial cultures as seen by changes in gene expression measured by RT-qPCR. Relative quantification (RQ) for each marker is shown relative to actB and TBP housekeeping genes and normalized with respect to undifferentiated organoids grown in IntestiCult OGM (Human). Progenitor markers (A) Lgr5 and (B) Axin2 are significantly reduced in both submerged monolayers and ALI cultures, while markers of enterocytes (KRT20, C), goblet cells (MUC2, D), and enteroendocrine cells (CHGA, E) are significantly increased. Further reduction in Axin2 is seen in ALI monolayers with an increase in expression of KRT20, MUC2, and CHGA. (F, G) Comparing cross-sections of organoid monolayers grown in IntestiCult™ ODM as (F) submerged culture or (G) at the ALI shows further differentiation of the intestinal epithelium with an increased proportion of goblet cells and extracellular mucus (MUC2, green).
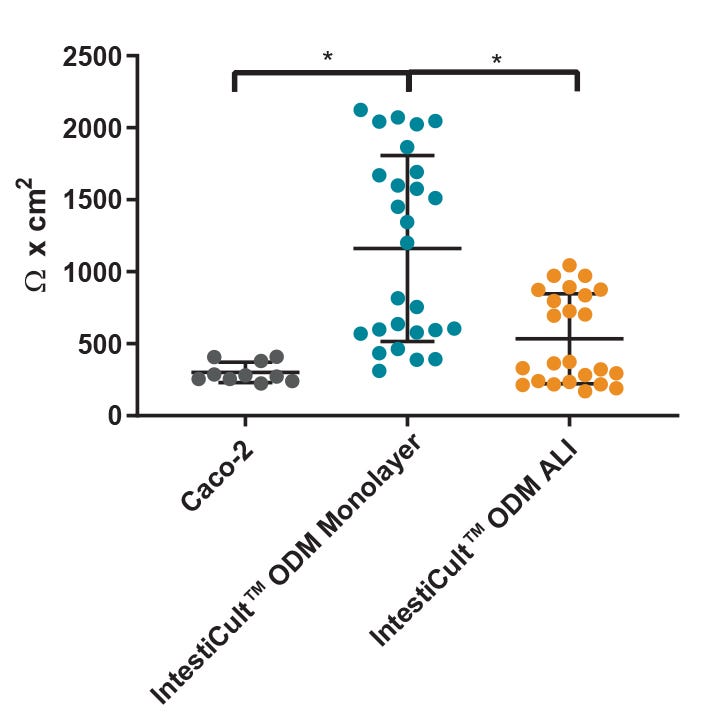
Figure 4. Differentiated Organoid-Derived Monolayers and ALI Cultures Display More Physiological Trans-Epithelial Electrical Resistance (TEER) than Caco-2 Cells
Differentiated organoid-derived monolayers grown as a submerged monolayer (IntestiCult™ ODM Monolayer), or at the ALI (IntestiCult™ ODM ALI), show higher TEER values as compared to Caco-2 cultures.Organoid-derived monolayers grown at the ALI show a loosening of tight junctions due to further differentiation of the brush border, and thus lower TEER values are observed. * p < 0.0001.
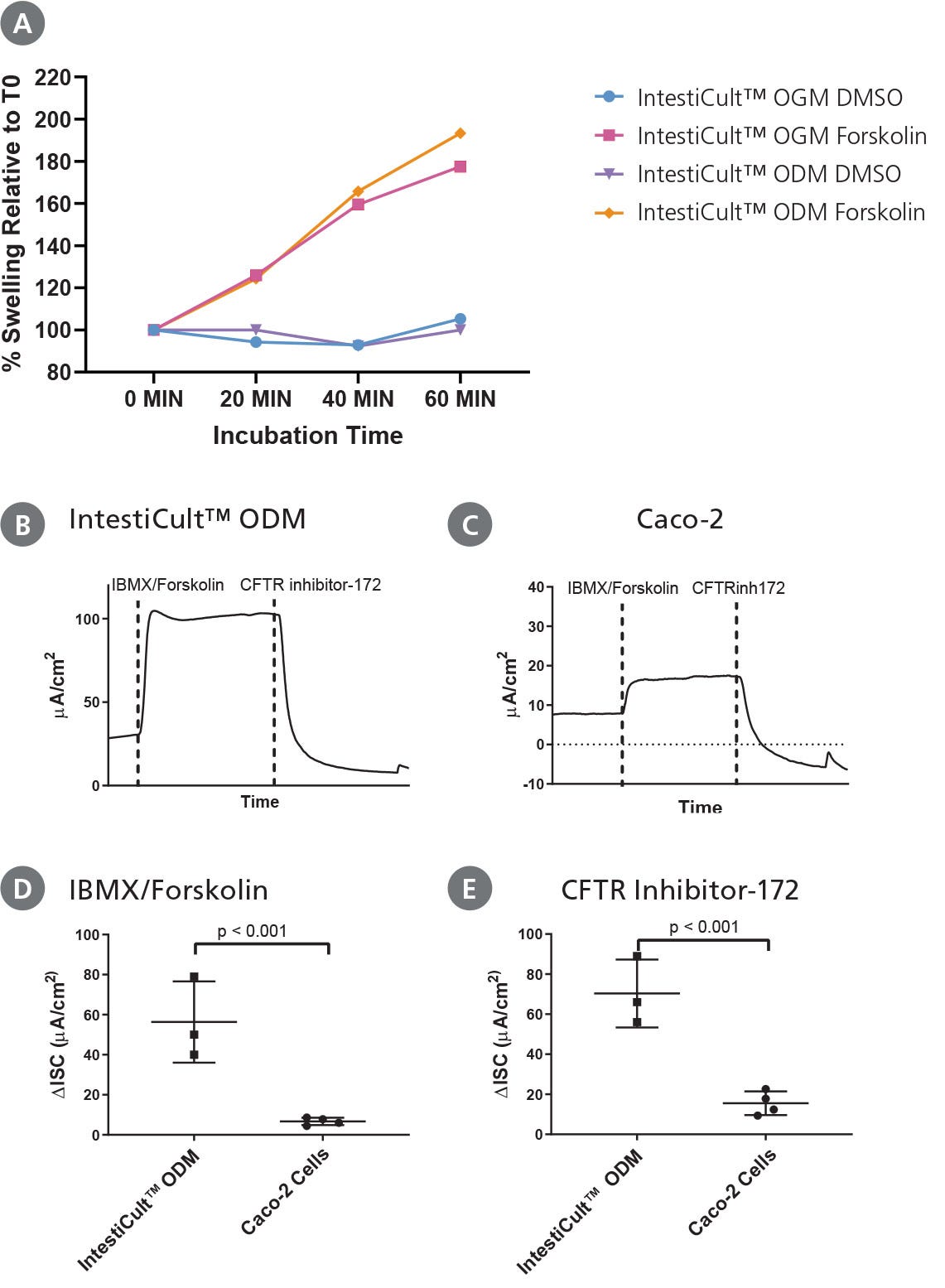
Figure 5. Differentiated Intestinal Organoids Provide a Suitable Model for Studying CFTR Response In Vitro
(A) Organoids differentiated further in IntestiCult™ ODM show a comparable degree of swelling when treated with forskolin as compared to organoids grown in IntestiCult™ OGM, demonstrating suitability for use in forskolin-induced swelling assays. (B – E) Ussing chamber analysis of submerged (B) organoid-derived monolayers and (C) Caco-2 cultures demonstrate increased sensitivity of organoid-derived monolayers to CFTR activation and inhibition by IBMX/Forskolin and CFTR Inhibitor-172 respectively. (D, E) Analysis of CFTR modulation by IBMX/Forskolin and CFTR Inhibitor-172 show significantly greater (D) activation and (E) inhibition of CFTR activity in organoid-derived monolayers as compared to Caco-2 cultures (p < 0.001 for both).

Figure 6. The MIMETAS OrganoReady® Colon Organoid Platform Uses IntestiCult™ to Create an Advanced Physiologically Relevant Model for Gastrointestinal Toxicity Testing and Barrier Integrity
(A) The OrganoReady® plate highlighting the microfluidic compartments.
(B) Schematic of the OrganoReady® microfluidic compartments where columns 1, 2, and 3 house the medium, a collagen-1 matrix, and the colon organoid tubule, respectively.
(C) Immunofluorescence staining of the colon organoid tubule confirms an adult tissue phenotype with the presence of goblet cells (Muc2), enterocytes (Occludin), and stem cells (Sox9). The 3D-lumenized structure provides apical (Ezrin) and basolateral (Integrin-β4) access to the polarized epithelium. Additionally, the organoid tubules show polarized and modulatable activity of expression of P-glycoprotein (Pgp).
(D) The OrganoReady® Colon Organoid platform supports toxicity testing, as demonstrated by dose-dependent measurements of TEER, LDH, and ATP following exposure to Afatinib (n = 4, N = 2). After 72 hrs of exposure, a dose dependent decrease in TEER, cytotoxicity, and cell viability was observed. For more information, please visit mimetas.com/en/organoready-organoid/.
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Resources and Publications
Educational Materials (52)
Related Products
-
 Trypsin-EDTA (0.05%)
Trypsin-EDTA (0.05%)Enzymatic cell dissociation reagent
-
 DMEM/F-12 with 15 mM HEPES
DMEM/F-12 with 15 mM HEPESDulbecco's Modified Eagle's Medium/Nutrient Ham's Mixture F-12 (DMEM/F-12) with 15 mM HEPES buffer
-
 D-PBS (Without Ca++ and Mg++)
D-PBS (Without Ca++ and Mg++)Dulbecco’s phosphate-buffered saline without calcium and magnesium
-
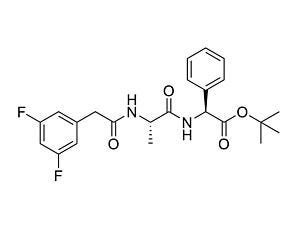 DAPT
DAPTNotch pathway inhibitor; Inhibits γ-secretase
Item added to your cart

IntestiCult™ Organoid Differentiation Medium (Human)
This product was developed under a license to intellectual property owned by Hubrecht Organoid Technology (HUB). This product is sold for research use only. Purchase of this product does not include the right to use it for drug screening aiming for commercial gain, equipment validation, biobanking, or for other commercial purposes. Purchasers wishing to use the product for purposes other than basic research use should contact HUB at www.huborganoids.nl to obtain a further license. Purchasers may apply for a License from HUB, which will not be unreasonably withheld by HUB.
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.
CA WARNING: This product can expose you to Progesterone which is known to the State of California to cause cancer. For more information go to www.P65Warnings.ca.gov




















































