Organoid Mini-Symposium: Human Intestinal Organoids to Study Rare Gut Endocrine Cells
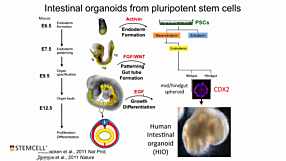
Joep Beumer, from Hans Clevers’ lab at the Hubrecht Institute, discusses how intestinal organoids can be used to study the molecular makeup of human gut enteroendocrine subtypes and the regulated secretion of individual hormones. He also features his lab’s work on successfully infecting intestinal organoids with SARS-CoV-2.
This webinar was part of our Organoid Mini-Symposium, held on April 22, 2020, which featured speakers using organoids in a variety of downstream applications.
Joep Beumer, from Hans Clevers’ lab at the Hubrecht Institute, discusses how intestinal organoids can be used to study the molecular makeup of human gut enteroendocrine subtypes and the regulated secretion of individual hormones. He also features his lab’s work on successfully infecting intestinal organoids with SARS-CoV-2.
This webinar was part of our Organoid Mini-Symposium, held on April 22, 2020, which featured speakers using organoids in a variety of downstream applications.
Webinar Q&A
Below is a list of questions submitted by participants during the recording of the webinar. Answers are provided by Joep Beumer (JB), with Ayse Guven (AG) as the moderator. 1. AG: You have shown beautiful data related to organoids and the induced differentiation of enteroendocrine cells with NEUROG3. Did you still maintain stem cells within these organoids or was this an endpoint differentiation, and how long were these organoids viable? JB: That’s a good question! In principle, all the stem cells were lost. Around 70 - 80% will become enteroendocrine cells and then the others will become goblet cells or other secretory cells. This is an endpoint differentiation and they will stay alive for about two weeks maximum. The differentiation is optimal at day 4 but they will stay alive for another week or even a bit longer. 2. AG: How was the infection performed for the SARS-CoV-2 experiments? Did you use microinjection and, related to this, do you think that the infectivity of SARS-CoV-2 would be different in reversed polarity organoids? JB: We broke the organoids by mechanical dissociation before bringing them in contact with the virus. For differentiated organoids it’s a bit more challenging. We used TrypLE to help dissociate and bring them into small clumps, then we brought them into contact with the virus. Finally, they were then re-associated and re-plated. For flip polarity, I think it would work. However, we have just used mechanical dissociation. 3. AG: You have shown beautiful data related to organoids and the induced differentiation of enteroendocrine cells with NEUROG3. Did you still maintain stem cells within these organoids or was this an endpoint differentiation, and how long were these organoids viable? JB: I guess it would really depend on the application, I am mostly working on cell fate choices. For many organoid systems there are one or more cell types that are lacking due to certain culture conditions, and for several applications this is a challenge. I believe a solution would be figuring out which signals guide the specification of the different lineages, to optimize that, and tailor organoids for research questions. That’s how I would go about it as someone who mostly works on cell fate.
Publish Date:
May 04, 2020
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
By submitting this form, you are providing your consent to STEMCELL Technologies Canada Inc. and its subsidiaries and affiliates (“STEMCELL”) to collect and use your information, and send you newsletters and emails in accordance with our privacy policy. Please contact us with any questions that you may have. You can unsubscribe or change your email preferences at any time.