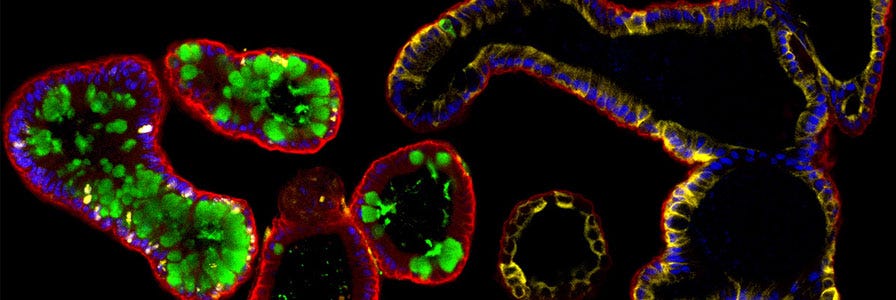
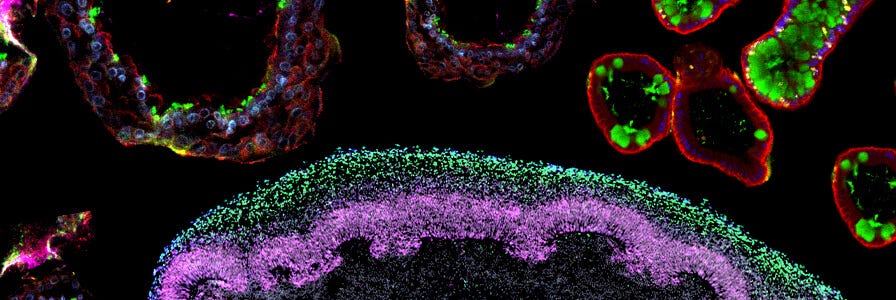
FAQs on Human Intestinal Organoid Cultures
Find answers to frequently asked questions (FAQs) about culturing human intestinal organoids using IntestiCult™.
Culture Initiation
Does Human IntestiCult™ only work with colonic biopsies?
Human IntestiCult™ works with both biopsies and surgical samples from the human colon and small intestine. While IntestiCult™ is optimized for colonic samples, as these are often easier to obtain, it works equally well with small intestinal tissue. For tissue from surgical samples, please note that a different protocol is necessary for initial isolation of intestinal crypts for culture.
How can I prevent clogging of the strainer during crypt isolation?
It is important to mince the sample thoroughly before treatment with Gentle Cell Dissociation Reagent (GCDR). Using either a scalpel or surgical scissors, you should cut the sample into small fragments before incubating in GCDR. More thorough tissue digestion will reduce clogging of the strainer and enable a greater number of stem cells to pass through the filter.
Does Human IntestiCult™ work with colon cancer biopsies?
Because of the heterogeneity of cancers, these samples may not all require the same medium composition for optimal growth. For instance, some colon cancers present mutations in the Wnt signaling pathway, one of the key signaling pathways that drive organoid maintenance. In these cultures, the medium composition required may be different from what we currently know and have designed Human IntestiCult™ for. If working with Wnt-independent samples, this technical bulletin can be used.
What size biopsies should I use?
We typically receive biopsies between 3 and 5 mm in diameter. Biopsies must be fresh (taken within 24 hours); frozen samples have low viability and are not recommended. Fresh biopsies can be stored in DMEM/F12 in an ice pack for transport. Although the size of the biopsy often varies, we suggest using ~5 mg of tissue. If the biopsies are much smaller, ensure that Y-27632 (RHO/ROCK pathway inhibitor/ROCki) is added.
Why are my primary cultures developing slowly?
Primary cultures can be slow to develop as some biopsies break apart easily, forcing organoids to start from single cells. In these cases, the organoids will develop, but will need about 3 weeks to do so. Once you have a handful of large, mature organoids, passaging should allow the culture to rapidly expand.
Can organoids be formed using single-cell seeding?
Lgr5+ stem cells derived from a human tissue sample and seeded into a Matrigel dome should be able to grow organoids. ROCKi will need to be added to the Human IntestiCult™ medium until the organoids are established, and it will take longer for organoids to reach maturity compared to using fragments. The same is true for primary colon cancer organoids.
Are there any differences in the tissue processing protocol between healthy and tumor biopsies?
Because tumor biopsies are generally tougher than healthy tissue biopsies, it may be necessary to physically break them up with a scalpel or razor during the tissue processing step. It may also be necessary to incubate tumor biopsies with Gentle Cell Disociation Reagent (GCDR) for longer than specified in the tissue processing protocol, to ensure sufficient dissociation of cells.
Passaging
How long can human organoid cultures be passaged?
In-house, we have maintained organoid cultures past passage 25; however, organoids may undergo subtle shifts in phenotype over time and prolonged passaging should be treated with caution. Passaging limits are highly donor-dependent and donor-to-donor variability is to be expected.
What is the ideal number of crypts (fragments) per dome when passaging?
After a dome has been broken up (Step 14 of the passaging protocol in the Product Information Sheet), 1000 crypts (broken up fragments) should be added to 1 dome, which will result in 150 - 200 mature organoids.
Can I plate fewer fragments per dome to create an environment with fewer dead cells?
The number of fragments per aliquot will likely vary between users. You should aim to obtain 150 - 200 organoids per dome. However, if you find plating less than 1000 fragments (or 150 organoids) results in cleaner or more viable cultures, you can try this, but please be aware that these cultures may not have optimal growth.
Does the protocol change if using human small intestine instead of colon?
No, the protocol remains the same.
Which area of the intestinal tract should I harvest from to obtain the greatest organoid yield?
Organoid growth may differ depending on where in the intestinal tract the crypts are obtained. For example, the small intestine contains Paneth cells, which help maintain the stem cell niche, while Paneth cells are not located in the colon. However, because there is substantial variation between human donors, it is difficult to generalize that increased proliferation/expansion is unique to one region of the intestine. We have had variable results from different donors from both regions of the intestine.
My organoids are not dissociating well. Do you have any suggestions?
If organoids are not dissociating at all, or large clumps remain, incubate for longer and/or pipette/vortex for longer to dissociate. Small clumps are acceptable and will not pose a problem. Having only single cells is not necessary.
Is it possible to transition IntestiCult™ Organoid Growth Medium (Human; 06010) cultures to IntestiCult™-SF Organoid Growth Medium (Human; 100-0214) for specific applications (or vice versa)?
Yes, it is possible to transition human intestinal organoid cultures maintained in IntestiCult™ Organoid Growth Medium (IntestiCult™ OGM) into IntestiCult™-SF Organoid Growth Medium (IntestiCult™-SF). It is important to note that you may see a shift in morphology once you switch the IntestiCult™ OGM cultures to IntestiCult™-SF. Typically, organoids in IntestiCult™-SF are more budded than those in IntestiCult™ OGM, but this can vary according to the donor. This transition will gradually take place over the first 1 - 2 passages in IntestiCult™-SF, and will not affect the expansion capacity of the culture.
Differentiation
What should I do if I'm having issues with cell attachment when performing the intestinal monolayer protocol?
This may be an issue with certain samples/donors. The best options are to increase the number of cells/organoids being used to seed the monolayers (to overcome the loss in efficiency with numbers), or to increase the matrigel concentration to 5% or even 10%.
The IntestiCult™ Organoid Differentiation Medium protocol recommends using organoid lines for up to 15 passages. Could higher passage numbers be problematic for attachment?
The passage number is one of the more likely reasons for attachment problems. Attachment efficiency decreases steadily as the passage number increases. This could be due to cells slowly shifting their gene expression from passage to passage, until they lose their ability to differentiate and attach to a surface. Hence, for optimal performance/differentiation, we recommend using samples at no later than passage 15 whenever possible.
Please note that this can be donor dependent and some donors may become problematic at lower passage numbers, while others remain fine long past P15.
What happens if the monolayer does not reach confluence within 2 - 3 days?
If the monolayer is not confluent after a few days (2-3 days), but cells are continuing to proliferate, we suggest monitoring for a few extra days, or up to an extra two weeks in an extreme case. However, if there is no noticeable cell attachment after 2-3 days, no sign of proliferation, or the monolayer has started to fall apart, there is likely no recovering this culture and you will need to start again.
I can't see any mucus layer forming in my ALI cultures. Do you have any suggestions?
The mucus layer is likely dehydrated. Rinse the apical chamber with a small volume of PBS and gently aspirate. Adding PBS will hydrate the secreted mucus.
There is a buoyant mucus layer when trying to dissociate organoids for the monolayer protocol. Can I remove it?
For some cultures/donors, it is possible to get a more differentiated phenotype during the expansion stage. There may be some goblet cells present, which produce mucus. This mucus layer tends to be buoyant, preventing the pellet from settling and making it difficult to break up the pellet. In this case, we suggest repeating the pipetting step to resuspend the cells, and centrifuging the sample again to help break up the mucus layer.
What is the purpose of adding Rho Kinase Inhibitor (ROCKi/Y-27362) when performing the monolayer protocol?
Rho kinase inhibitor is used primarily to keep cells alive as single cells during monolayer culture. Without it, most cells would become apoptotic during this step. After the monolayer reaches confluence, ROCKi becomes less necessary and can be removed from the medium. It does, however, have a beneficial purpose in slowing down anoikis (a form of programmed cell death that occurs in anchorage-dependent cells when they detach from the surrounding extracellular matrix), reducing the numbers of cells lost from the monolayer through this process. This will keep the monolayer confluent and stable for longer periods of time.
How long can the 2D monolayers be kept in the IntestiCult™ Organoid Differentiation Medium?
The 2D submerged and ALI monolayers can be maintained for at least 3 weeks with regular media changes. It is important to note that the differentiated monolayers are meant for endpoint assays.
Is there an ideal window or time point to collect TEER readings for the intestinal monolayers?
TEER readings are typically taken after 7 days for submerged monolayers in the interest of consistency between cultures and experiments; however, we have collected TEER readings consistently between 2 days and 12 weeks post seeding. The TEER increases as the culture reaches confluence and a few days beyond confluence, but then usually settles down to a stable value. Naturally, this can vary between cultures and any disruptions in the monolayer can change the TEER readings significantly. Usually, however, the TEER value remains relatively consistent.
Can't find the answer you are looking for? Reach out to us and one of our intestinal specialists will get back to you.
Intestinal Organoids Learning Center
Learn how organoids are being used to model the intestine in this collection of protocols, webinars, and other scientific resources.
Try STEMCELL’s Organoid Media for Intestinal Research
Request information about introductory offers to try STEMCELL’s organoid media in your own lab.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration