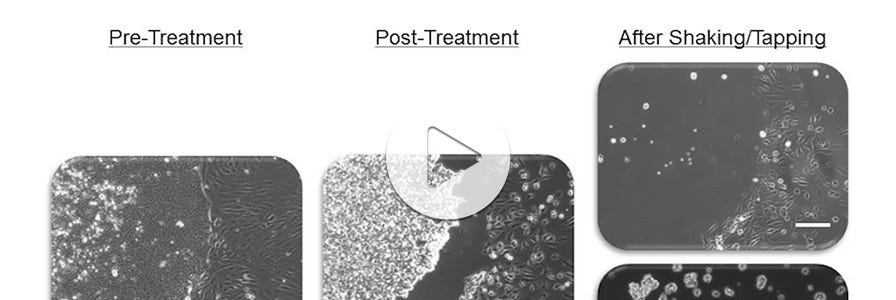
How Your Cell Culture Conditions Impact Pluripotent Stem Cell Quality

Pluripotent cells exist only transiently during embryogenesis. In 1998, James Thomson was the first to report culture conditions that could indefinitely capture a human pluripotent state. The maintenance and propagation of human embryonic stem (ES) cells and human induced pluripotent stem (iPS) cells requires the use of optimized media formulations, in combination with careful handling techniques, to maintain high-quality cultures at each passage.
Early methods to maintain human ES cells required the use of extensively screened sera and murine embryonic feeder cells. These undefined components often lead to inconsistent culture quality and challenges in reproducing results within and between groups. Use of feeder-free conditions eliminates the inherent biological variability of feeder cells. Efforts were then focused on improving the reproducibility of human ES and iPS cell culture protocols by simplifying and removing undefined components from all aspects of the culture system: media, matrices and passaging reagents. Recently, researchers have also investigated capturing earlier embryonic states resembling the pre-implantation blastocyst.
Although many researchers have adopted a more defined culture environment with the use of feeder-free matrices and defined culture media, one commonality is that all human pluripotent stem cell (hPSC) cultures, no matter the environment, are prone to acquiring genetic abnormalities in a non-random and sporadic manner. For now the exact mechanisms remain unknown, however new technologies have allowed us to better assess the quality of cultures, and to reduce stressors in the extracellular environment.
Tips for hPSC Culture
- Tech Tip Sticking Together - Clump Passaging for ES and iPS Cell Maintenance
Learn how passaging hPSCs as aggregates enables the long-term expansion of cell lines while maintaining a normal karyotype, without ROCK inhibitor. - Tech Tip Ensuring Optimal pH of hPSC Culture Medium
Learn about the impact of culture pH on hPSCs and cell culture best practices to prevent DNA damage due to acidosis.
Key Publications
- Jacobs et al. describe the link between medium pH and genomic instability of pluripotent stem cells.
Jacobs K et al. (2016) Higher-density culture in human embryonic stem cells results in DNA damage and genome instability. Stem Cell Reports. 6(3): 330–41 - Liu et al. describe the benefits of medium buffering on pluripotent stem cell culture.
Liu W et al. The suppression of medium acidosis improves the maintenance and differentiation of human pluripotent stem cells at high density in defined cell culture medium. Int J Biol Sci. 14(5): 485–496 - Wilmes et al. demonstrate that iPSC are sensitive to extracellular acidification.
Wilmes A et al. (2017) Towards optimisation of induced pluripotent cell culture: Extracellular acidification results in growth arrest of iPSC prior to nutrient exhaustion. Toxicol In Vitro 45(3): 445-454 - Ludwig et al. report the development of TeSR1, a defined, serum-free and animal product-free medium that supports the derivation and long-term feeder-free culture of human embryonic stem cells.
Ludwig T et al. (2006) Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 24(2): 185-7.
On-Demand Webinars
Development of Simplified and Defined Cell Culture for Human Pluripotent Stem Cells
Human pluripotent stem cells (hPSCs) have the ability to generate all cell types in the human body and can be used in many applications in basic research and translational medicine, including disease modeling, drug screening and cell therapy. Maintenance of high quality hPSCs is dependent on consistent in vitro cell culture conditions and handling techniques. TeSR™-E8™ is a defined, xeno-free medium with a simple and published formula that was developed to meet the demands of a wide range of applications. The E8 culture system has been successfully used to derive and maintain hPSCs, and enzyme-free methods have also been developed for cell expansion and cryopreservation. More recently, defined differentiation conditions have also been established for specific lineages. These developments will provide an efficient and cost-effective E8 platform for the hPSC field.

University of Macau, China
- Human pluripotent stem cells
- Defined cell culture for basic and clinical applications
- Efficient stem cell handling practices
- hPSC differentiation
The University of Sheffield
Dr. Guokai Chen obtained his PhD in Biochemistry and Molecular Biology from Baylor College of Medicine in Houston, Texas and continued onto a postdoctoral fellowship at the University of Wisconsin in the lab of Dr. James Thomson. Here, he contributed to the advancement of cell culture technologies of human pluripotent stem cells (hPSCs) by creating the defined E8 medium system for hPSC derivation and maintenance. Guokai also developed an EDTA-based enzyme-free method for hPSC culture. Guokai was a founding director of the induced Pluripotent Stem Cell (iPSC) and Genome Engineering Core Facility at the National Heart, Lung and Blood Institute where his research group contributed to the establishment of NIH control iPSC lines, high-throughput iPSC derivation, and cardiac differentiation methods. More recently, he has joined the Faculty of Health Sciences at the University at Macau.
mTeSR™3D for Expansion and Scale Up of Human Pluripotent Stem Cell Cultures
Dr. Eric Jervis, Principal Scientist at STEMCELL Technologies, introduces mTeSR™3D for suspension culture of human pluripotent stem cells. This video was filmed during the Innovation Showcase at ISSCR 2016 in San Francisco.
Scalable Enzyme-Free Protocols for the Isolation and Maintenance of hiPSCs Without Mechanical Colony Scraping
Dr. Erik Hadley, Senior Scientist at STEMCELL Technologies, gives a tutorial focusing on fibroblast reprogramming and hiPSC maintenance protocols that use ReLeSR™, an enzyme-free passaging reagent that eliminates manual removal of differentiated cells, colony scraping and complicated techniques to obtain uniform cell aggregates.
More Resources
Video: A Guide to Passaging Human Pluripotent Stem Cells Using mTeSR™1
mTeSR™1 is a defined medium designed for the maintenance of hESCs and hiPSCs without the use of feeders. This video describes the morphology of cells at different stages during culture and also describes the method to passage cells cultured in mTeSR™1.
Video: Limitless Potential: Do More with TeSR™
mTeSR™1 is a highly specialized and defined, serum-free and complete cell culture medium, with established protocols for applications ranging from gene editing, bioreactor expansion, to lineage-specific differentiation.
Tools for High-Quality hPSC Culture
TeSR™-E8™
Feeder-free, animal component-free culture medium for maintenance of human ES and iPS cells
Vitronectin XF™
Defined, xeno-free matrix that supports the growth and differentiation of human pluripotent stem cells
CellAdhere™ Laminin-521
Matrix for maintenance of human ES and iPS cells in combination with mTeSR™1, TeSR™-E8™, or TeSR™2