Ensuring Optimal pH of hPSC Culture Medium
Technical tip from our dedicated team of Product and Scientific Support specialists
Environmental stressors such as reactive oxygen species (ROS), nutrient exhaustion, and build-up of metabolites such as lactic acid have been investigated for their potential contribution to DNA damage, which in turn may increase the likelihood of genetic abnormalities.1,2
Interestingly, acidification of the extracellular environment to pH below 7.0 has been shown to attenuate glycolytic rates,1 and below 6.9 has been correlated with significant increases in DNA damage.2 Moreover, reducing medium acidity alone has been shown to reduce apoptosis in culture.3 hPSCs are best maintained within the physiological pH range — between 7.2 and 7.4.4 Historically, the preferred way to ensure a healthy extracellular environment is to provide cells with fresh media on a daily basis to remove waste products and replenish nutrients. More recently, advancements in medium buffering enable pH to remain stable for up to 72 hours, even at high cell densities, without the need for a medium change.
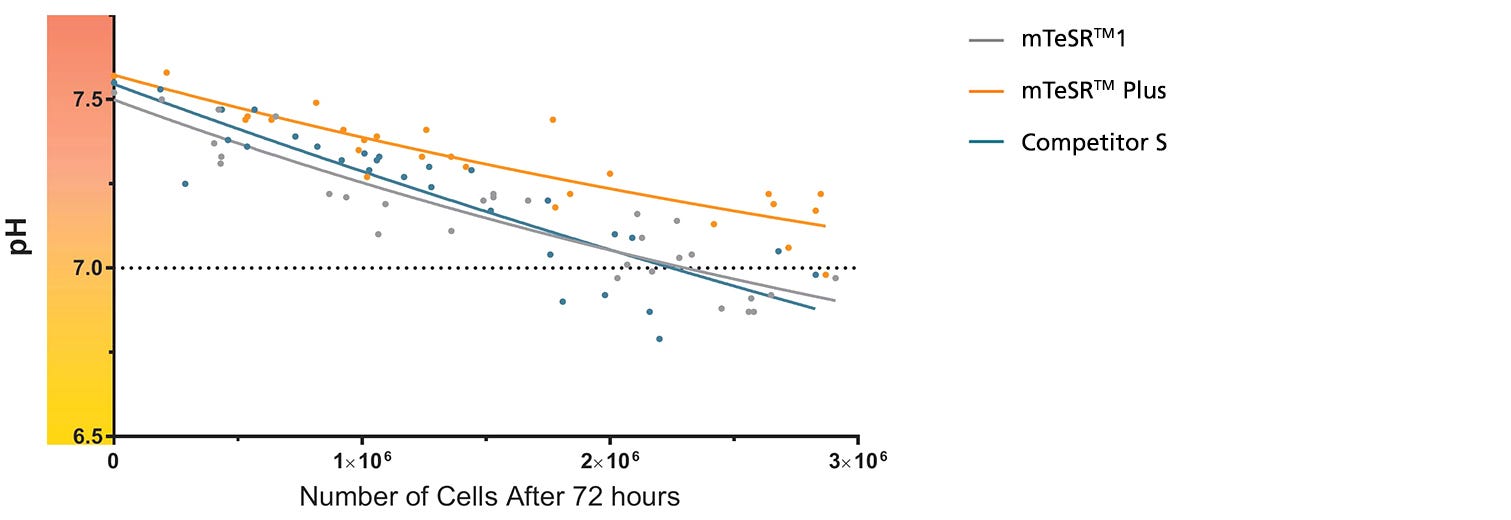
Figure 1. mTeSR™ Plus Maintains Optimal pH Levels Throughout a Weekend-Free Protocol
The pH of spent medium from hPSCs cultured in mTeSR™ Plus is higher than that of hPSCs cultured in mTeSR™1 and other flexible-feeding medium at similar cell densities. pH and cell numbers were measured after a 72-hour period without feeding. Range of cell numbers shown represent different densities that would be observed throughout a typical passage. This demonstrates that feeds can be skipped for two days at any time during routine maintenance using mTeSR™ Plus while maintaining a pH above 7.0. Note: Cultures were fed double the standard medium volume prior to the 72-hour period without feeds in all media and cell numbers are from one well of a 6-well plate.
For further assistance and information, please contact techsupport@stemcell.com.
References
- Wilmes A et al. Toxicol In Vitro 45(3): 445–454, 2017
- Jacobs K et al. Stem Cell Reports. 6(3): 330–341, 2016
- Liu W et al. Int J Biol Sci. 14(5): 485–496, 2018
- Ludwig T et al. Nature Biotechnol. 24(2): 185–7, 2006
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration

