Hematopoietic Stem and Progenitor Cells
For Your Cell-Based Assays
What are Hematopoietic Stem and Progenitor Cells?
Hematopoietic stem cells (HSCs) are arguably one of the most well-characterized stem cell populations owing to their long history of research (Weissman et al., 2000). HSCs make up a very small population of the hematopoietic system, however, these cells are invaluable as they have the potential to give rise to all mature blood and immune cell types and sustain life-long blood production. Also making up a part of the hematopoietic system are short-term repopulating progenitor cells, which give rise to lineage-specific cell types. Collectively, these are referred to as hematopoietic stem and progenitor cells (HSPCs).
HSCs specifically are defined by their ability for self-renewal and to reconstitute the entire hematopoietic system following transplantation. This property forms the basis of in vivo assays of HSC function. In experimental settings, the ability of human HSCs to reconstitute hematopoiesis is measured by transplantation into genetically immunocompromised mice (e.g. NOD-SCID-Gamma (NSG) mice) and measuring the presence of human blood cells in the blood or bone marrow (BM) after extended periods of engraftment, e.g., 20 weeks or longer.
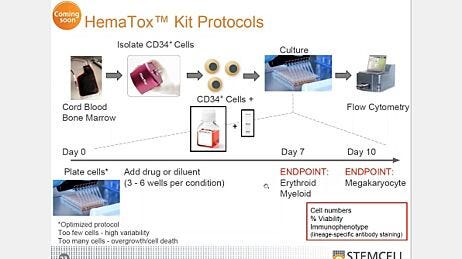
The colony-forming unit (CFU) assay is a clonal, in vitro culture assay that measures the growth and frequency of functionally viable HSPCs by assessing the proliferation and differentiation of individual progenitor cells, resulting in the formation of discrete colonies in a semi-solid methylcellulose medium (such as MethoCult™) when supplemented with appropriate cytokines.
Colonies derived from different types of progenitor cells are classified and counted based on morphological and phenotypic criteria. The CFU assay was first described by Metcalf et al., 1979 and today is the most widely used in vitro assay for HSPCs. It is used both by hematopoietic researchers and clinical labs to assess the potency of transplantation units of cord blood (CB) and hematopoietic cellular therapy products. Importantly, the CFU assay has been shown to correlate well with the successful engraftment of HSPCs (Page et al., 2011).
Other uses of the CFU assay include studying the effects of stimulatory and inhibitory growth factors, screening novel compounds to predict potential toxicity to the hematopoietic system, and testing the effects of various in vitro manipulations (e.g. cell processing, cryopreservation, gene transduction, and transmission) on cellular products used in hematopoietic cell transplantation.
What is CD34?
Cell markers, or surface antigens, are molecules located on a cell’s membrane used to identify specific cell types, their lineage, and their stage in the differentiation process according to the presence or absence of the expression of defined markers. CD34 is a transmembrane phosphoglycoprotein that was first identified on HSPCs, but its expression is also present on other cell types, such as vascular tissue.
CD34 is the most commonly used cell surface marker to identify human HSPCs as it is expressed on HSCs, in addition to both multipotent and more differentiated progenitor cells of individual blood cell lineages. Additional markers can be used to distinguish HSPC subsets within the CD34+ population and isolate HSPCs with different engraftment abilities and capacities to expand or generate mature blood cells in culture.
For example, HSCs can be enriched to relatively high purity by isolating the ~1% of CD34+ cells that don't express mature blood markers (lineage-negative) and are CD38-, CD45RA-, and CD90+. In one study, as few as 10 Lin-CD34+CD38-CD45RA-CD90+ CB cells were needed to successfully engraft into the BM of immunodeficient mice, with the generation of human lymphoid and myeloid cells seen 12 weeks following engraftment (Majeti R et al., 2007). Additional markers, e.g. CD49f, EPCR, and CD133, have been used to further purify HSCs or identify primitive HSPCs after ex vivo culture (Notta et al., 2011).
Figure 1 shows images of CFU colonies generated from HSPCs defined by CD34 expression and quantified using STEMvision™—an instrument designed for automating and standardizing the CFU assay.
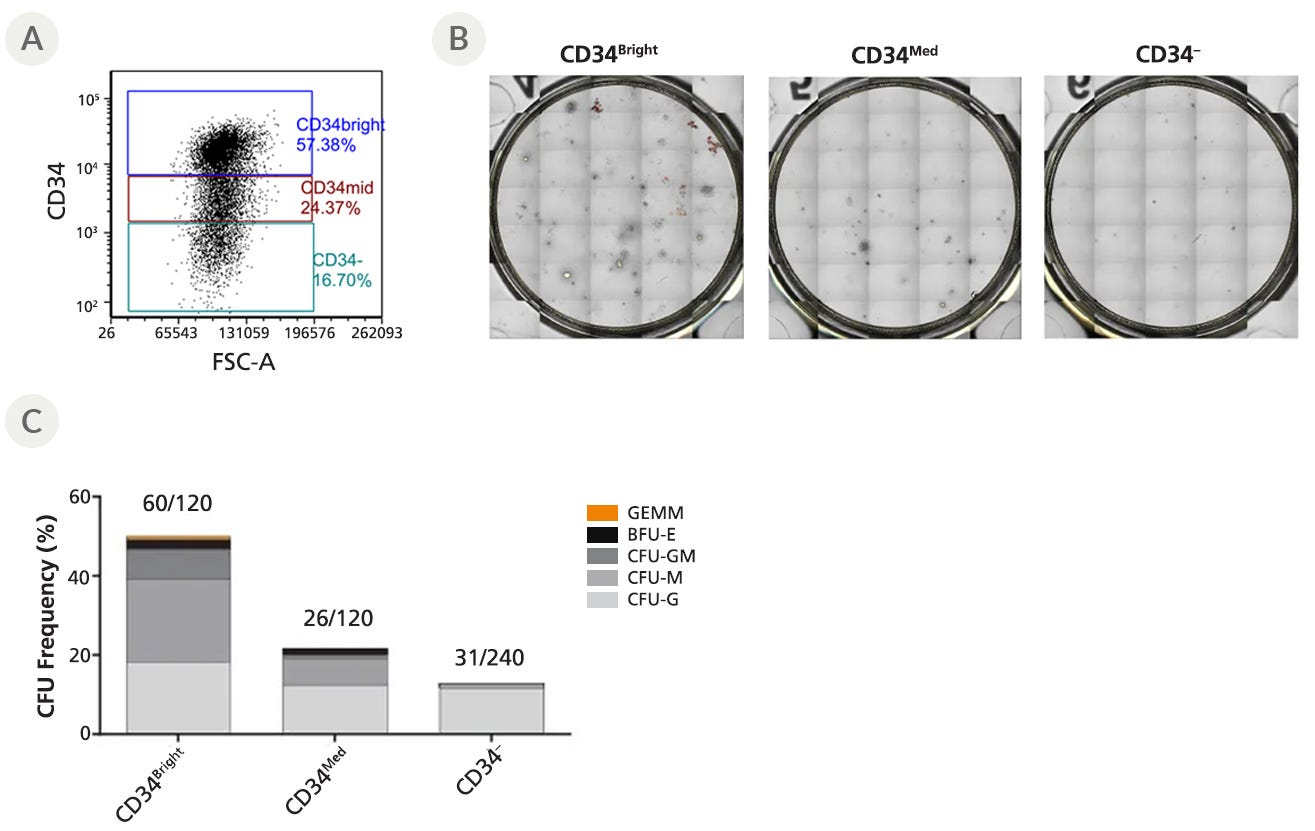
Figure 1. CFU Frequency of Sorted CD34+ Cells
CD34+ CB cells were cultured (n = 2) in StemSpan™-XF with StemSpan™ CD34+ Expansion Supplement and UM171 for 7 days. Cultured cells were then stained with an antibody against CD34 and CD34high, CD34med, and CD34negative cells were bulk- or single-cell sorted directly into the CFU assay. (A) Representative flow cytometry plot showing CD34 expression. (B) Representative images of CFU colonies generated from bulk-sorted cells, counted with STEMvision™. Bulk-sorted cells were plated at 400 cells/mL in 6-well SmartDish™ plates (Catalog #27370). (C) CFU frequency of single sorted cells with distinct levels of CD34+ expression. The culture-expanded CD34bright cells showed higher CFU frequency (~50%) than CD34med cells (~20%) or CD34- cells (~10%). GEMM: granulocyte, erythrocyte, monocyte, megakaryocyte, BFU-E: burst forming unit-erythroid, CFU-GM: colony-forming unit-granulocyte-macrophage, CFU-M: colony-forming unit-megakaryote, CFU-G: colony-forming unit-granulocyte.
Learn more about hematopoiesis, the assays used to identify and quantify human HSPCs, and how to identify and count colonies within the CFU assay with our on-demand CFU assay training course.
Tissue Sources of HSPCs
HSPCs reside primarily in the bone marrow (BM), but small numbers can also be found in peripheral blood (PB) and other tissues in adults, as well as in the cord blood (CB) of infants, and thus can be obtained from a variety of sources. HSPCs can be mobilized from the BM and released into the PB after the injection of cytokines, specifically granulocyte colony-stimulating factor (G-CSF) and/or other agents. For mobilization, donors can be mobilized using specific regimens with the following mobilizing agents:
- G-CSF - The cytokine G-CSF is the most commonly used mobilizing agent and is considered a gold standard in the clinic
- Plerixafor - The bicyclam molecule plerixafor is a rapid-acting mobilization agent
- Combination - The combination of G-CSF and plerixafor works synergistically, increasing CD34+ mobilization compared to single-agent mobilization
Mobilized PB (mPB) HSPCs can be collected by apheresis and are currently the key source of HSPCs for clinical transplantation to treat hematologic malignancies and other blood cell disorders. Figure 2 shows the expansion data for mPB-derived CD34+ cells cultured in StemSpan™ media compared to commercial alternatives.
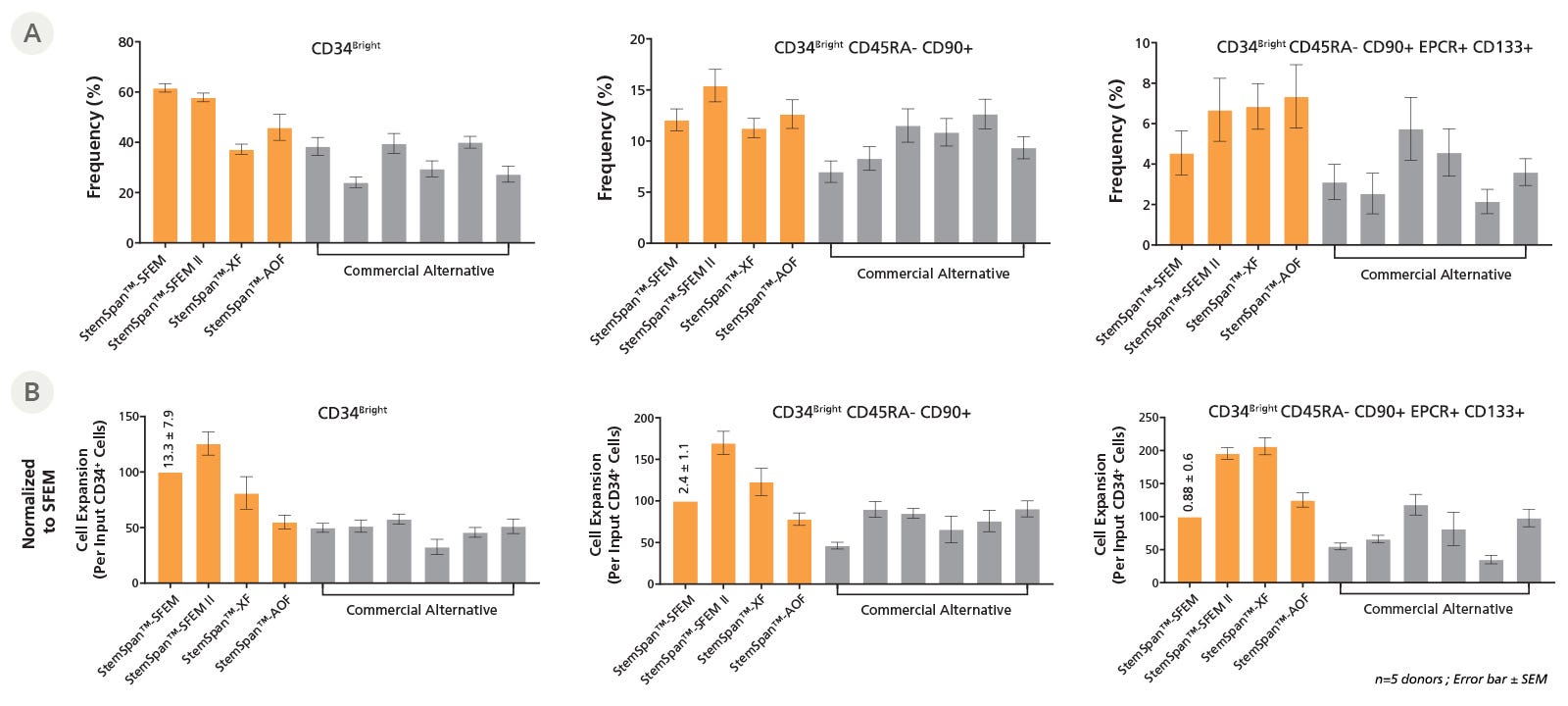
Figure 2. StemSpan™ Media Supports Equal or Greater Expansion of Mobilized Peripheral Blood HSPCs Compared to Other Commercial Media
Purified CD34+ cells derived from G-CSF mobilized peripheral blood (mPB) were cultured at a concentration of 10000 cells per mL in StemSpan™ media (SFEM, SFEM II, AOF, and XF, orange bars), and in six media from other suppliers (Commercial Alternatives, grey bars). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM729 (1 uM). After 7 days of culture, the (A) frequency and (B) cell expansion of viable CD34bright, CD34bright CD45RA-CD90⁺, and CD34bright CD45RA-CD90⁺CD133⁺EPCR⁺ cells were analyzed by flow cytometry, as described in Figure 1. Fold expansion was normalized to StemSpan™ SFEM (the number above the bar indicates average yield per input CD34+ cell ± SD). Compared to the competitor media tested, StemSpan™ media showed similar or higher expansion of CD34bright, CD34bright CD45RA-CD90+, and CD34bright CD45RA-CD90+CD133+EPCR+ cells (each subsequent cell subset being progressively more enriched for functional stem/progenitor cells). Data shown are mean ± SEM (n = 5).
Umbilical CB is also used as an alternative source of allogeneic HSPCs for patients for whom a suitable BM or mPB donor is not available. BM, mPB, and CB are also commonly used for basic research into hematopoietic stem cell biology and the development of novel cellular therapy applications, e.g. HSC expansion and gene therapy.
Table 1 outlines some of the advantages and disadvantages of the various sources of CD34+ cells. The most appropriate choice for your research will depend on a variety of factors, such as your application, research goals, and of course, the availability of cells from each source.
Table 1. CD34+ Cell Sources, Frequencies (%), and Features
- Higher frequency of CD34+ cells compared to the other two sources
- More representative than CB of the cell source used for HSC transplantation or gene therapy
- Less invasive procedure for collection when compared to BM
- More readily available for research, such as for basic HSC biology and proof-of-principle studies for cellular therapy development
- Limited donor volumes, meaning low cell numbers and variable quality of individual CBs
- Greater capacity for cell division than other CD34+ cell sources; ~85x increase in cell numbers over 10-day expansion
- Higher cloning efficiencies and proliferative capacity in comparison to adult sources, such as BM
- Larger initial sample sizes than CB
- Donors are recallable
- Additional cell types can be isolated, including mesenchymal stromal cells (MSCs) and mononuclear cells
- Limited number of CD34+ cells and lower proliferative potential for genetic manipulation
- Data sourced from STEMCELL Technologies
- These frequencies are expected when using fresh samples that are less than 48 hours old. After 48 hours, the cell frequency is expected to decline
- Sourced from: https://www.stemcell.com/technical-resources/cord-blood-cd34-cell-isolation.html
- Washed BM
Learn more about human HSPC phenotypes, frequencies, and hierarchies with this handy wallchart.
Why Choose HSPCs from STEMCELL Technologies?
STEMCELL Technologies provides a range of sources for researchers to obtain CD34+ cells from. Ensure continuity in your research and start experiments according to your schedule with a reliable supply of high-quality, donor-specific primary cells that are ready to use upon receipt, with guaranteed viability and purity documented in the lot-specific Certificate of Analysis (CoA).
Learn more about how we can work closely with you as your primary cells supplier.
Why Use HSPCs from STEMCELL Technologies?
- Choose cells that are more physiologically representative of cells in vivo.
- Access donor samples collected using regulatory authority-approved consent forms and protocols.
- Request custom products for non-standard cell types or collections with specific requirements.
- Reserve large numbers of cryopreserved cells and start experiments on your schedule with cells you've already tested.
- Reduce time spent collecting and culturing primary cells.
Cord Blood CD34+ Cells
Use human CD34+ stem and progenitor cells that have higher proliferative capacity and cloning efficiencies.
View Product >Bone Marrow CD34+ Cells
Start with a large initial sample size with CD34+ stem and progenitor cells from adult human BM.
View Product >Peripheral Blood CD34+ Cells
Streamline your assays by using frozen mobilized primary CD34+ cells.
View Product >Mobilized Peripheral Blood Leukopak, Fresh, G-CSF*
Obtain large numbers of fresh single-donor CD34+ HSPCs from PB leukopaks mobilized with G-CSF.
View Product >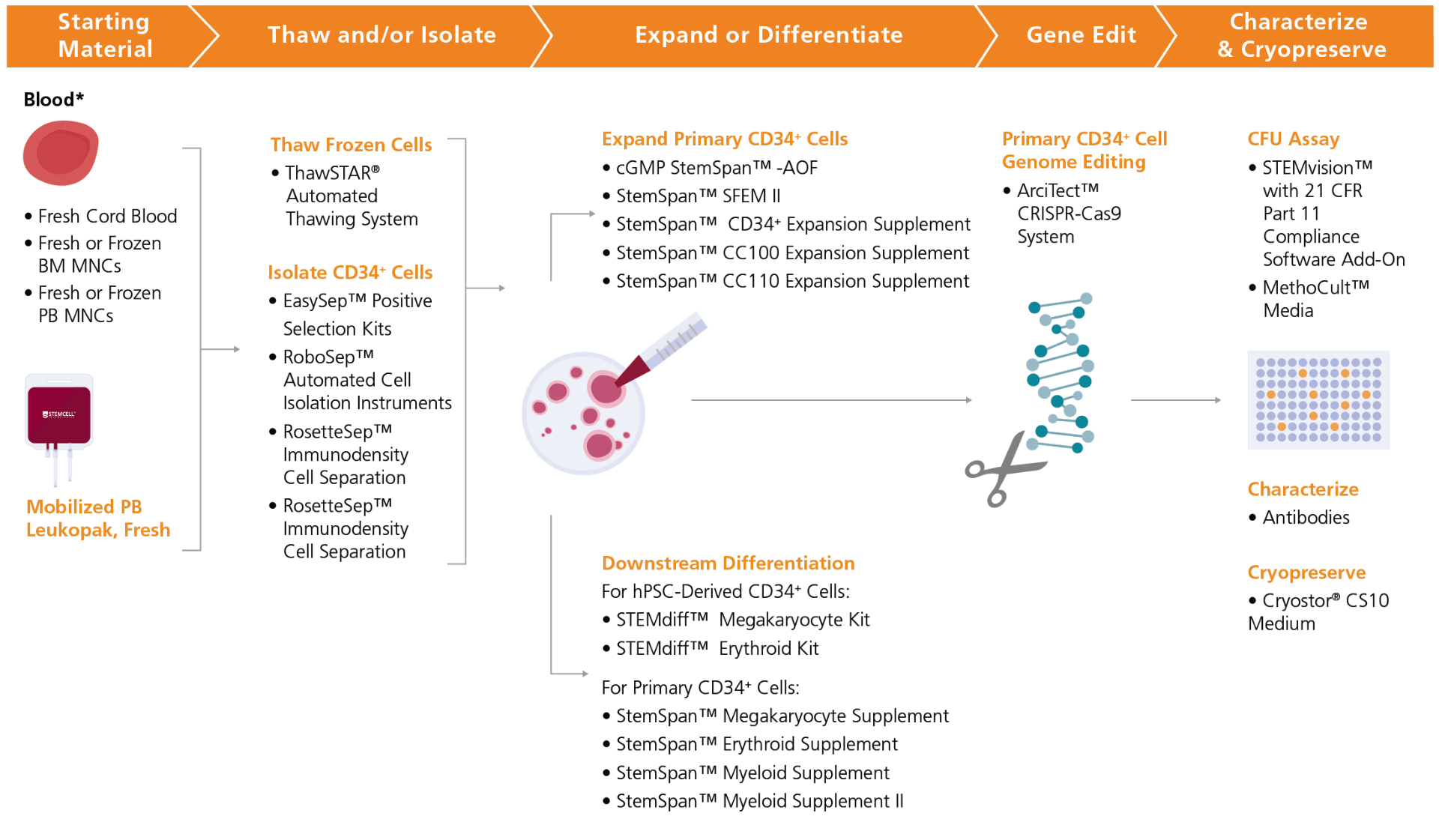
Figure 3. Complete Product Workflow for Hematopoietic Cell and Gene Therapy Research
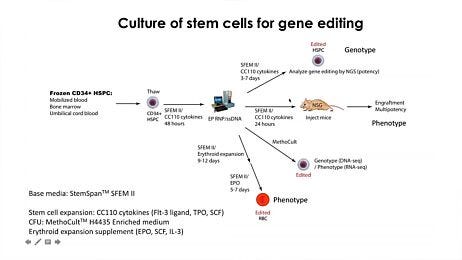
Start with a reliable source of HSPCs by using our fresh or frozen human blood products, including MNCs. CD34+ or other cell subsets may be isolated from these samples by using our immunomagnetic EasySep™ cell isolation kits. Alternatively, you may start with our ready-to-use human primary CD34+ cells. Human CD34+ cells can be reproducibly expanded or differentiated in serum-free conditions with StemSpan™ media and supplements—such as cGMP, animal origin-free StemSpan™-AOF medium—or with lineage-specific STEMdiff™ kits. Primary cell-derived CD34+ cells may be efficiently gene-edited using the ArciTect™ CRISPR-Cas9 System. Unmodified and gene-edited CD34+ cells can be cultured in MethoCult™ media and analyzed using the STEMvision™ instrument, which is now available with a software add-on for use in high-compliance environments. BM MNCs: bone marrow-derived mononuclear cells, ES Cells: embryonic stem cells, PB MNCs: peripheral blood-derived mononuclear cells.
Applications of HSPCs
HSPCs have many applications in and out of the clinic. The following are some of the main areas where HSPCs are used in research:
- Humanized mouse generation
- Hematological disease research
- Cancer and other disease modeling
- Cell and gene therapy
- Transplantation and engraftment studies
- Drug screening and toxicity testing
- Basic research into HSPC function, mobilization, and differentiation
- Research into the bone marrow stem cell niche
- Novel mobilization regimens for hematopoietic stem cell transplantation
Standardize Your Downstream Cell Processes
Explore some of our cell processing technologies and resources to support the standardization of your cell culture workflows.
Cell Thawing Instrument
Standardize your cell thawing process with the ThawSTAR® CFT2 or ThawSTAR® CB Automated Thawing System—sensor-based, water-free instruments for a standardized thawing process.
View Products >Specialized Cell Culture Media and Supplements for HSPCs
Promote the expansion of HSPCs or encourage their lineage-specific differentiation, and analyze your cells with media and supplements from our StemSpan™, MethoCult™, and MyeloCult™ portfolios.
View Products >Cell Isolation Kits
Isolate cells such as CD34+ cells from CB and achieve high purity and recovery with our EasySep™ cell isolation kits.
View Products >Cell Separation Guide E-Book
Learn everything you need to know about cell isolation techniques by downloading our cell separation e-book.
Get Your Free Copy >Resources for Your Cell-Based Assays
Find webinars and technical resources to help you work with hematopoietic cells in your research below.
Find out how we can help you get to where you need to be in your HSPC research:
Proficiency Testing Programs
Assess your ability to perform the CFU assay and identify areas for improvement. You will be provided with the cells, reagents, and instructions required to perform the assay and your results will be analyzed and compared to other participants according to ISO 13528 guidelines.
Our Assay Services Workflow
- Preparation of a proposal that clearly defines project scope, timeline and cost.
- Experimental execution and data analysis.
- Preparation of final report.
References
- Weissman et al. Cell Press (2000) 100,157-168
- Metcalf D et al. J. Cell. Physiol. 98(2), 401-420 (1979)
- Page et al. Biol Blood Marrow Transplant (2011) 17(9) 1362-74
- Majeti et al. Cell Stem Cell (2007) 1(6) 635-45
- Notta et al Science (2011) 8;333(6039):218-21
*Certain products are only available in select territories. Please contact your sales representative or the Product & Scientific Support team at techsupport@stemcell.com for further information.