Expansion and Differentiation of Hematopoietic Stem and Progenitor Cells into Natural Killer (NK) Cells Using StemSpan™ Medium and Supplements
- Document # DX22912
- Version 1.0.3
- May 2024
Why Use StemSpan™ for Generating NK Cells?
- Serum- and feeder-free conditions eliminate variation introduced by serum and stromal cell lines.
- Produce approximately 9,000 NK cells per input CB-derived CD34+ cell.
- Eliminate extra passaging steps required with stromal cell-based cultures.
Background
Natural killer (NK) cells are lymphocytes that play an important role
in innate immunity due to their ability to secrete proinflammatory
cytokines as well as kill cancerous or virus-infected cells. Human
NK cells are derived from CD34+ hematopoietic stem and progenitor
cells (HSPCs) in vivo. The ability to expand CD34+ HSPCs in vitro, and promote their differentation into NK cells is a useful tool for research
into the use of NK cells for adoptive immunotherapy in cancer patients
as well as research into basic NK cell biology.
The StemSpan™ NK Cell Generation Kit (Catalog #09960) facilitates
the differentiation of CD34+ HSPCs from cord blood (CB) or bone
marrow (BM) into NK cells without the use of stromal cells, and in
serum-free culture conditions.
StemSpan™ NK Cell Generation Kit (Catalog #09960)
*Also available for individual sale.
**500 mL format is also available (Catalog #09655).
Note: The addition of UM729 (Catalog #72332) is strongly recommended for success of this protocol. UM171 may also be used, but the concentration must be adjusted. See PIS for more details. These products are not included in the StemSpan™ NK Cell Generation Kit and must be purchased separately.
NK Cells Generated with the StemSpan™ NK Cell Generation Kit
Medium and Supplements for the Expansion and Differentiation of HSPCs into NK Cells
The StemSpan™ NK Cell Generation Kit contains serum-free medium and coating material, as well as expansion and differentiation supplements (see table below) that promote the generation of NK cells from HSPCs in a two-step protocol. In the first step, CF34+ HSCPCs are cultured for 14 days in medium containing expansion supplement to stimulate their proliferation and differentiation into lymphoid progenitor cells. In the second step, these lymphoid progenitor cells are cultured for another 14 days in medium containing the differentiation supplement and the UM729 (Catalog # 72332; sold separately) to promote their further differentiation into CD56+ NK cells. UM729 is necessary during this second phase of the cultures to achieve high NK cell frequencies and yields. In this system, approximately 9,000 NK cells can be generated per input CB CD34+ cell (see Figure 2). Both fresh and cryopreserved CB- and BM-derived CD34+ cells may be used with the StemSpan™ NK Cell Generation Kit.
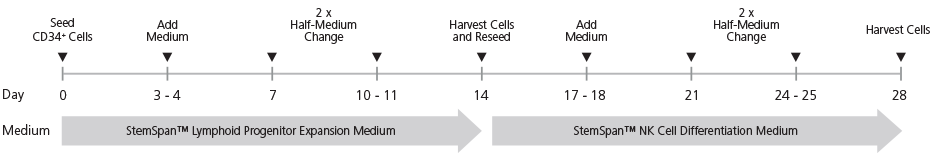
Figure 1. StemSpan™ NK Cell Generation Protocol
CB-derived CD34+ cells are seeded on day 0. Medium should be topped up after 3 - 4 days of culture followed by two half-medium changes every 3 - 4 days. On day 14, cells at the lymphoid progenitor stage are harvested and reseeded for further differentiation into NK cells. Top-up and half-medium changes should be performed every 3 - 4 days after harvest and reseed, as indicated in the figure. Note: UM729 should only be added to the NK Cell Differentiation Medium (see step 6), but not the Lymphoid Progenitor Expansion Medium. For further details see the protocol below.
Protocol
This protocol is designed to promote the proliferation and
differentiation of CB-derived CD34+ cells into CD56+ NK cells over 28 days of culture. See Figure 1 for an overview of this protocol. This procedure has been optimized for use with CB-derived CD34+ cells. If using BM-derived CD34+ cells, refer to the Product Information
Sheet (PIS; Document #DX22777) for complete instructions.
Note: Optimal cell yields depend on maintenance of proper cell
health, which largely depends on following the recommended
schedule of feeding and medium changes.
- Coat non-tissue culture-treated plates with StemSpan™ Lymphoid Differentiation Coating Material; refer to the PIS for complete instructions.
- Thaw frozen CB-derived CD34+ cells (Catalog #70008), or isolate CD34+ cells from fresh CB using EasySep™ Human Cord Blood CD34 Positive Selection Kit II (Catalog #17896).
- Prepare StemSpan™ Lymphoid Progenitor Expansion Medium (StemSpan™ SFEM II + StemSpan™ Lymphoid Progenitor Expansion Supplement).
- Dilute CD34+ cells to 1 x 104 cells/mL in Lymphoid Progenitor Expansion Medium and seed into the precoated plate.
- Incubate at 37°C for 14 days, following instructions in the PIS for required half-medium changes. Lymphoid progenitor cells are harvested on day 14 for further differentiation to NK cells.
- Prepare StemSpan™ NK Cell Differentiation Medium (StemSpan™ SFEM II + NK Cell Differentiation Supplement + UM729).
- Dilute lymphoid progenitor cells to 1 x 105 cells/mL in StemSpan™ NK Cell Differentiation Medium. Seed into a non-coated tissue culture plate, incubate at 37°C, and follow instructions in the PIS for required half-medium changes.
- On day 28, harvest cells (containing CD56+ NK cells; see Figure 2) for use in downstream assays.
Applications
- Research into differentiation of NK lineage cells from HSPCs
- Assessment of efficacy and toxicity of candidate therapeutics on NK cell differentiation during drug development
- Research into the use of NK cells for potential development of cellular therapeutics
- Development of in vitro models to study diseases that involve NK cells
Cell Analysis
The protocol described in this technical bulletin can be used to generate CD56+ NK cells after 28 days of culture of CB-derived CD34+ cells. The differentiated cells can be stained with antibodies directed against cell surface markers CD56, NKp46, NKp44, NKp30, NKG2D, CD94, CD16, and KIR and analysed using flow cytometry (see Figures 2A and 3 for representative flow cytometry plots). Shown below are flow cytometry plots, yields, and frequencies generated using the protocol. In addition to cell surface marker expression, the average frequency and yield of CD56+ NK cells generated in culture are shown (Figure 2B).
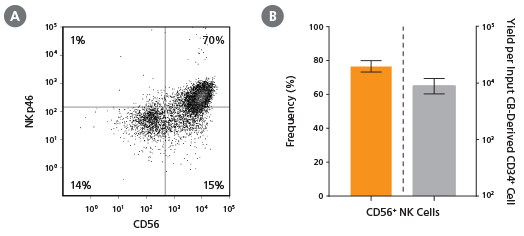
Figure 2. Frequency and Yield of CD56+ NK Cells After 28 Days of Culture
CB-derived CD34+ cells (freshly isolated or frozen) were cultured with the StemSpan™ NK Cell Generation Kit for 28 days as described. Cells were harvested and analyzed for (A,B) CD56 and (A) NKp46 expression by flow cytometry. Dead cells were excluded by light scatter profile and viability staining. (B) The average frequency of viable CD56+ NK cells on day 28 was 77%, with ~9,000 CD56+ cells produced per input CB-derived CD34+ cell. Shown are means with 95% confidence intervals (n = 45: 23 freshly isolated and 22 frozen CD34+ cell samples). BM-derived CD34+ cells were also differentiated into NK cells using the StemSpan™ NK Cell Generation Kit. The yield of NK cells from BM HSPCs is typically lower than with CB, averaging ~75 per input CD34+ cell (n = 3, data not shown).
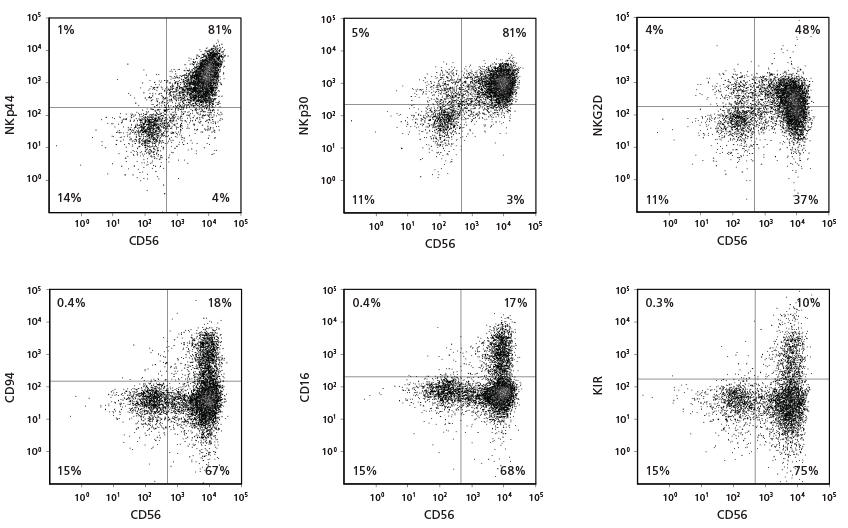
Figure 3. Cell Surface Marker Expression on CD56+ NK Cells After 28 Days of Culture
CB-derived CD34+ cells were cultured with the StemSpan™ NK Cell Generation Kit for 28 days. The differentiated cells were harvested and analysed by flow cytometry for the expression of CD56, NKp44, NKp30, NKG2D, CD94, CD16, and KIR. Staining for KIR molecules was performed using a combination of two clones for the antibody, 180704 and HP-MA4, as each recognizes a distinct subset of KIR molecules.
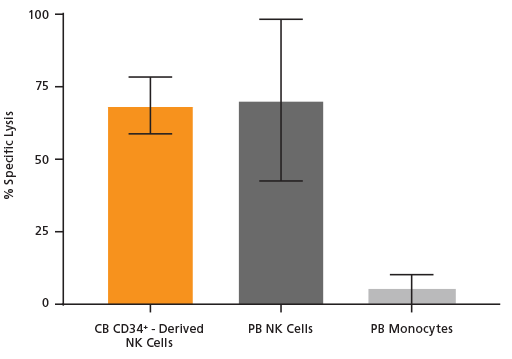
Figure 4. Cultured NK Cells Exhibit Cytotoxicity Toward K562 Cell Line
NK cells were generated from CB-derived CD34+ cells over 28 days using the protocol in Figure 1. On day 28, cells were harvested, stained for CD56, and viable CD56+ cells were counted. K562 cells were incubated with 8 μM calcein AM at 37°C for 1 hour and then washed twice. CD56+ NK cells were then combined with 10,000 of these calcein AM-labeled K562 target cells at an Effector:Target ratio of 5:1 in U-bottom 96-well plates and co-cultured at 37°C for 4 hours. Adult peripheral blood (PB) NK cells and monocytes isolated using EasySep™ were used as positive and negative controls, respectively. PB NK cells were cultured overnight with the NK Cell Differentiation Supplement and SFEM II, while PB monocytes were cultured overnight in SFEM II only. To detect spontaneous release, control wells containing only calcein AM-labeled K562 target cells were set up. The labeled K562 cells were treated with 1% Triton™ X-100 to measure maximum release. After incubation, plates were centrifuged at 500 x g for 5 minutes and 100 μL of supernatant was transferred to black plates and analyzed using a SpectraMax® microplate reader (excitation 485 nm/emission 530 nm). Results are expressed as % specific lysis: [(test release - spontaneous release) x 100] / (maximum release - spontaneous release). CB CD34+-derived NK cells show similar killing activity toward K562 target cells compared to PB NK cells. Shown are means ± SD (CB CD34+-derived NK cells: n = 18, PB NK cells and monocytes: n = 7).
Recommended Antibodies for Analysis*
*Not included in the kit.
Accessory Products*
*Not included in the kit.
†Available in select regions; contact your sales representative for further information.
Explore These Resources
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration