StemSpan™ Hematopoietic Stem Cell Media and Supplements
StemSpan™ Hematopoietic Cell Media and Supplements
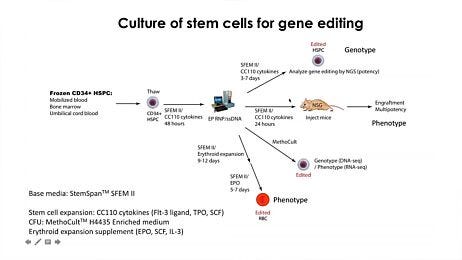
Reproducible Hematopoietic Stem and Progenitor Cell Expansion and Differentiation
StemSpan™ serum-free hematopoietic cell expansion media promote the expansion of normal or leukemic human hematopoietic stem and progenitor cells (HSPCs), or their lineage-specific differentiation when supplemented with hematopoietic growth factors and/or other stimuli selected by the user. StemSpan™ expansion supplements are pre-mixed cocktails of recombinant human cytokines and other additives formulated to selectively promote the expansion of CD34+ stem and progenitor cells, or to stimulate their differentiation into erythroid, myeloid (granulocyte or monocyte) or megakaryocyte progenitor cells, when added to StemSpan™ media. Complete kits, consisting of medium, supplements, and substrate, allow for the expansion and lineage-specific differentiation of CD34+ cells into T or NK cells in stroma-free conditions. Additionally, kits which combine medium, supplements, and small molecules allow for the culture and expansion of CD34+ cells isolated from chronic myeloid leukemia (CML) or acute myeloid leukemia (AML) patient samples.
Why Use StemSpan™ Media and Expansion Supplements?
- Components are carefully selected and screened to minimize lot-to-lot variability, consistently providing optimal culture conditions.
- Media do not contain cytokines, allowing the flexibility to add StemSpan™ Expansion Supplements, cytokines and/or additives.
- StemSpan™ SFEM II, combined with the appropriate Expansion Supplement, supports greater expansion of CD34+ cells and differentiation of erythroid cells, granulocytes, monocytes, and megakaryocytes, than other media tested.
- In addition to serum- and xeno-free formulations, StemSpan™-AOF is the first commercially available animal component-free medium for culturing HSPCs.
cGMP Hematopoietic Stem Cell Expansion Media
Think forward to the clinic when developing your hematopoietic cell and gene therapy research. cGMP StemSpan-AOF can help smooth your path through the regulatory landscape by removing animal-derived components from your hematopoietic cell culture conditions. StemSpan™-AOF contains only recombinant proteins and synthetic components and is manufactured and tested following relevant cGMPs under a certified quality management system. Find more information about regulatory compliance at STEMCELL >
- 20X expansion of human cord blood-derived CD34+ cells after 7 days of culture
- Maintenance of primitive CD34brightCD90+CD45RA- population in culture
- Suitable for use in genome editing protocols
- Animal component- and phenol red-free formulation
- Manufactured and tested under relevant cGMPs
- Full traceability of raw materials
Advantages:
Unsure Which StemSpan™ Formulation is Right for You?
Find the right StemSpan™ medium for your needs, whether it is expansion of CD34+ cells or their differentiation into erythroid cells, granulocytes, monocytes, and megakaryocytes.
Data
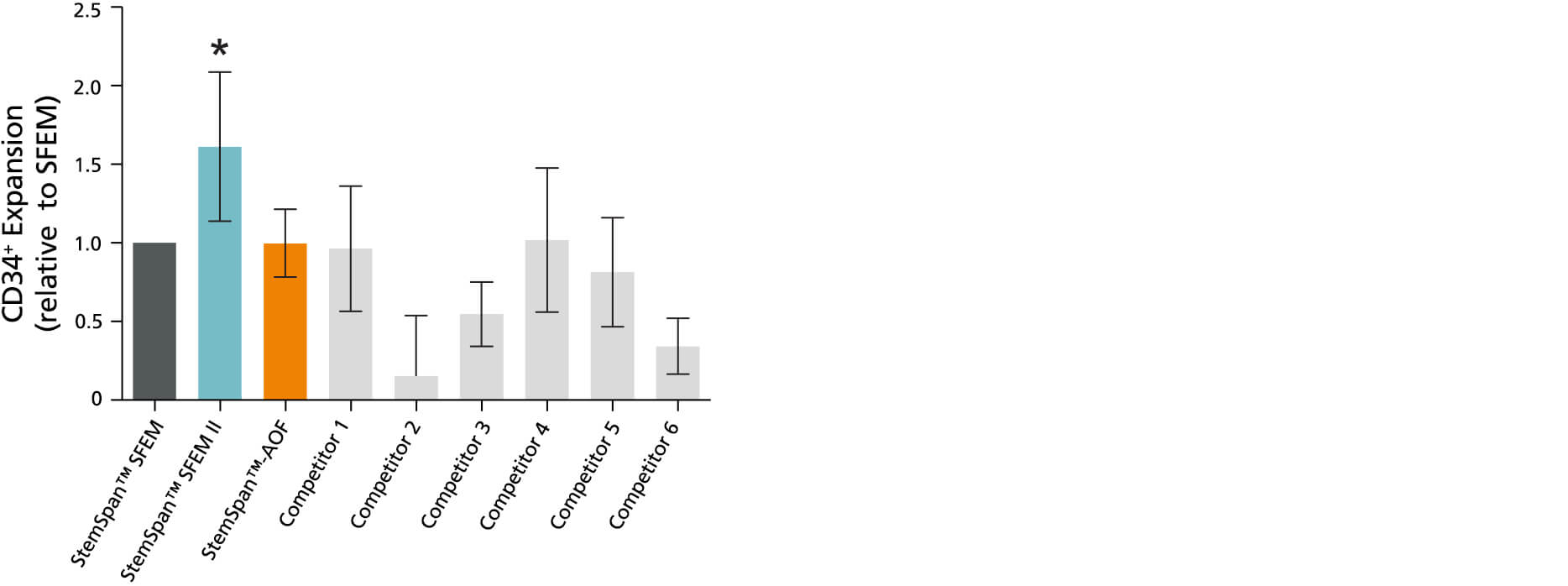
Figure 1. StemSpan™ SFEM II Serum-Free Expansion Medium Containing CC100 Cytokine Cocktail Supports Greater Expansion of Human CD34+ Cells Than Other Media Tested
Expansion of CD34+ cells normalized relative to the values obtained in StemSpan™ SFEM medium (dark gray bar) after culturing purified + CB cells for 7 days in StemSpan™ SFEM, SFEM II and AOF, and six media from other commercial suppliers (light gray bars; Competitor 1 - 6, which included, in random order, StemLine II (Sigma), HPGM (Lonza), HP01 (Macopharma), SCGM (Cellgenix), StemPro34 (Life Technologies) and X-Vivo-15 (Lonza)). All media were supplemented with StemSpan™ CC100 Cytokine Cocktail (Cat #02697). Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of cells produced in StemSpan™ SFEM II were significantly higher than in all other media, except the numbers of CB cells produced in StemSpan™-AOF (*p<0.05, paired t-test, n = 6).
Note: Data for StemSpan™-AOF shown were generated with the original phenol red-containing version (Catalog #09855). However internal testing showed that the performance of the new phenol red-free, cGMP-manufactured version of StemSpan™-AOF(Catalog #100-0130) was comparable.
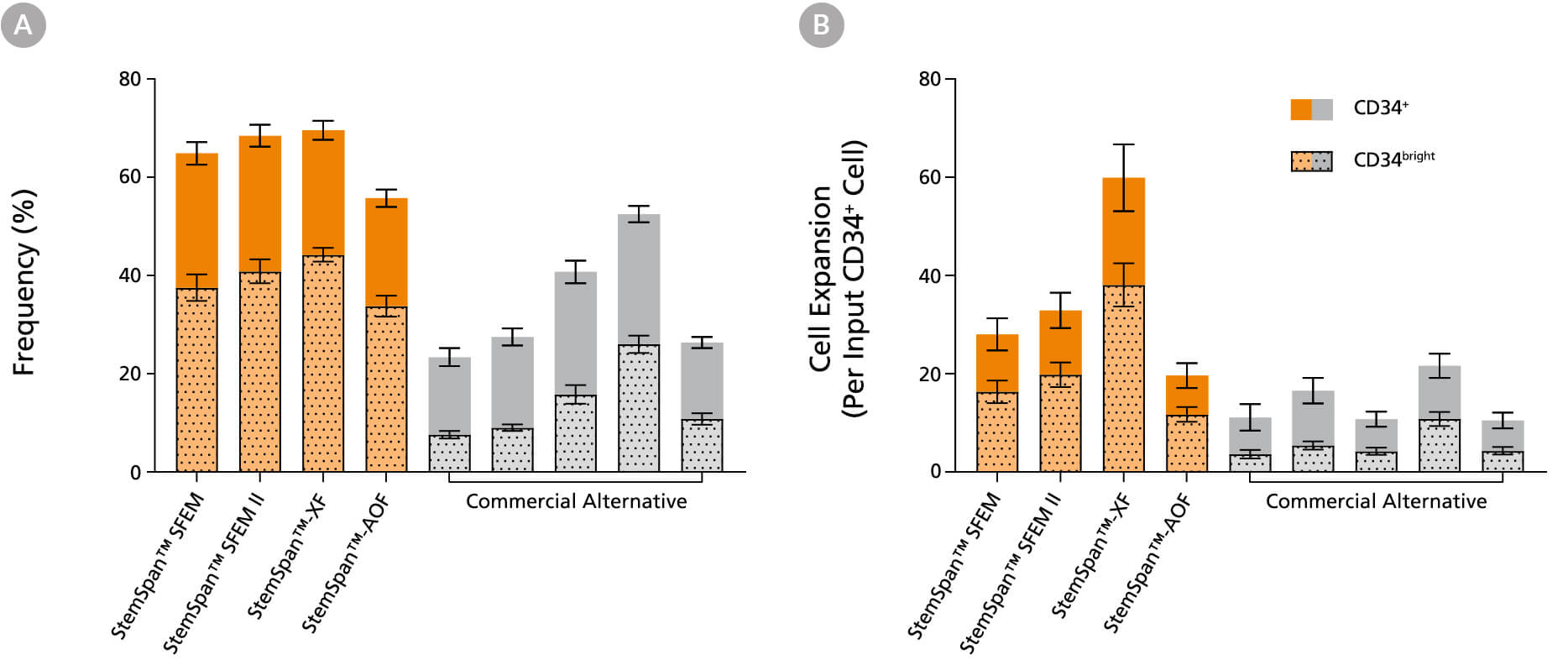
Figure 2. StemSpan™ Media Support Greater Expansion of Human CD34+ and CD34bright Cells Than Other Commercial Media
Purified CB-derived CD34+ cells were cultured for 7 days in StemSpan™ media (SFEM, SFEM II, AOF, and XF, orange bars), and in five media from other suppliers (Commercial Alternative, grey bars). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM171*. The (A) frequency and (B) cell expansion of viable CD34+ and CD34bright cells in culture were analyzed by flow cytometry as described in Figure 1. Compared to the commercial alternatives tested, StemSpan™ media showed significantly higher expansion of CD34+ and CD34bright cells (P < 0.05 when comparing StemSpan™ SFEM, SFEM II, and XF to five media from other suppliers, calculated using a one-way ANOVA followed by Dunnett’s post hoc test). The CD34bright cell population is enriched for functional stem/progenitor cells (See Figure 4). Data shown are mean ± SEM (n = 8).
*Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1μM. For more information including data comparing UM171 and UM729, see Fares et al. 2014.
Note: Data for StemSpan™-AOF shown were generated with the original phenol red-containing version (Catalog #09855). However internal testing showed that the performance of the new phenol red-free, cGMP-manufactured version of StemSpan™-AOF(Catalog #100-0130) was comparable.

Figure 3. Analysis of 7 Day Expanded Mobilized Peripheral Blood CD34⁺ Cells in StemSpan™ SFEM II medium by Flow Cytometry
Purified CD34+ cells derived from G-CSF mobilized peripheral blood (mPB) were cultured for 7 days in StemSpan™ SFEM II medium (Catalog #100-0130) supplemented with StemSpan™ CD34+ Expansion Supplement (Catalog #02691) and UM729 (Catalog #72332). After 7 days, the cultured cells were stained with fluorescently labeled antibodies against CD34, CD45RA, CD90, EPCR, and CD133, in addition to viability dye Zombie Yellow™, and analyzed by flow cytometry. The horizontal dotted line on the CD34 and FSC plot (left) indicates the fluorescence minus one (FMO) control for CD34 marker expression. Sequential gates (orange) were used to determine the percentages of viable CD34bright cells, CD34brightCD90⁺CD45RA⁻ cells and CD34bright CD45RA⁻CD90⁺CD133⁺EPCR⁺ cells presented in Figures 2, 3, and 4.

Figure 4. StemSpan™ Media Support Equal or Greater Expansion of Mobilized Peripheral Blood HSPCs Compared to Other Commercial Media
Purified CD34+ cells derived from G-CSF mobilized peripheral blood (mPB) were cultured at a concentration of 10000 cells per mL in StemSpan™ media (SFEM, SFEM II, AOF, and XF, orange bars), and in six media from other suppliers (Commercial Alternatives, grey bars). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM729 (1uM). After 7 days of culture, the (A) frequency and (B) cell expansion of viable CD34bright, CD34bright CD45RA⁻CD90⁺ and CD34bright CD45RA⁻CD90⁺CD133⁺EPCR⁺ cells were analyzed by flow cytometry, as described in Figure 1. Fold expansion was normalized to StemSpan™ SFEM ( number above bar indicates average yield per input CD34+ cell ± SEM). Compared to the competitor media tested, StemSpan™ media supported similar or higher expansion of CD34bright, CD34bright CD45RA⁻CD90⁺ and CD34bright CD45RA⁻CD90⁺CD133⁺EPCR⁺ cells (each subsequent cell subset being progressively more enriched for functional stem/progenitor cells). Data shown are mean ± SEM (n=5).

Figure 5. Estimation of Long-term SCID Repopulating Cell in Cord Blood CD34⁺ cells expanded in either StemSpan™-AOF or StemSpan™ SFEM II using Limiting Dilution Transplantation assay
Purified cord blood-derived CD34⁺ cells were cultured for 7 days in either StemSpan™-AOF or StemSpan™ SFEM II supplemented with StemSpan™ CD34⁺ Expansion Supplement and UM729 (1 μM). After 7 days of expansion, progeny of 10, 100, 250 and 2500 initial CD34⁺ cells were injected intravenously into sub-lethally irradiated NSG mice. For uncultured CD34⁺ cells, 250, 500 and 2500 cells were transplanted. The frequency of human cells expressing the pan-leukocyte marker CD45 was measured in bone marrow at ~20 weeks post-transplantation. A threshold of >0.1% of CD45⁺ cells was used to consider if mice were positive or negative for engraftment. Limiting dilution analysis was performed using the ELDA software from the Walter and Eliza Hall Institute of Medical Research software. LT-HSC frequencies (red lines) and 95% confidence intervals (boxes) are presented as 1/number of original CD34⁺ cell (day 0 equivalent) for each condition; n = 2 independent experiments performed, 2-7 mice per group per experiment. Significance level * p< 0.001 (Chi-square test). StemSpan™ SFEM II and StemSpan™-AOF expanded cells results in ~36 and ~17-fold increase in LT-HSC compared to fresh CD34⁺ cells, with SRC frequencies of 1/23, 1/48, 1/835 for StemSpan™ SFEM II, StemSpan™-AOF and fresh control respectively.
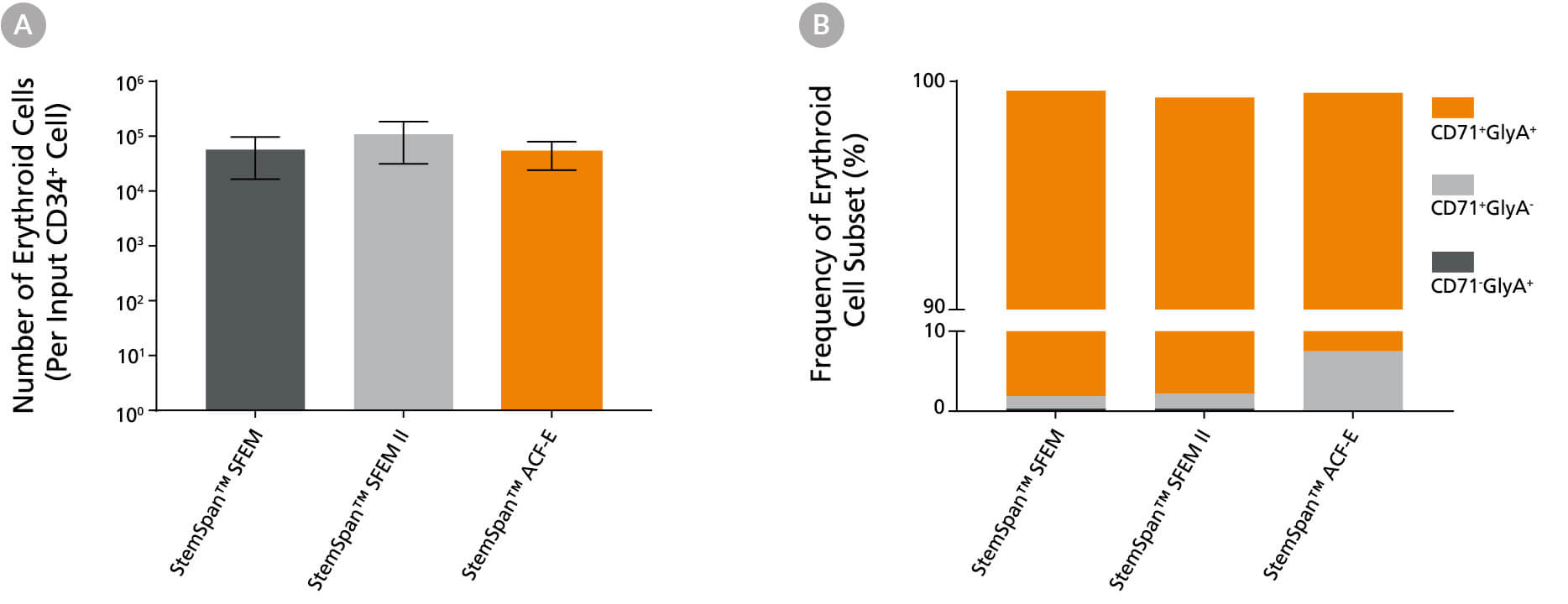
Figure 6. StemSpan™ SFEM II Serum-Free Expansion Medium Containing Erythroid Expansion Supplement Supports Greater Expansion of Erythroid Cells Than Other Media Tested
(A) Average numbers of erythroid cells generated after culturing purified CD34+ CB cells (n = 5) for 14 days in StemSpan™ SFEM (black bars), SFEM II (grey bars), or StemSpan™-ACF Erythroid Expansion Medium (ACF-E, orange bars) media containing StemSpan™ Erythroid Expansion Supplement (Catalog #02692). Shown are the number of erythroid cells that express CD71 and/or Glycophorin A (GlyA) per input CD34+ cell. (B) The percentages of the different erythroid cell subsets generated in these cultures are shown, including CD71+GlyA+ erythroblasts, immature CD71+GlyA- erythroid progenitor cells and pro-erythroblasts, and CD71-/lowGlyA+ normoblasts. All three media supported the generation of thousands of erythroid cells per CB-derived CD34+ cell plated.
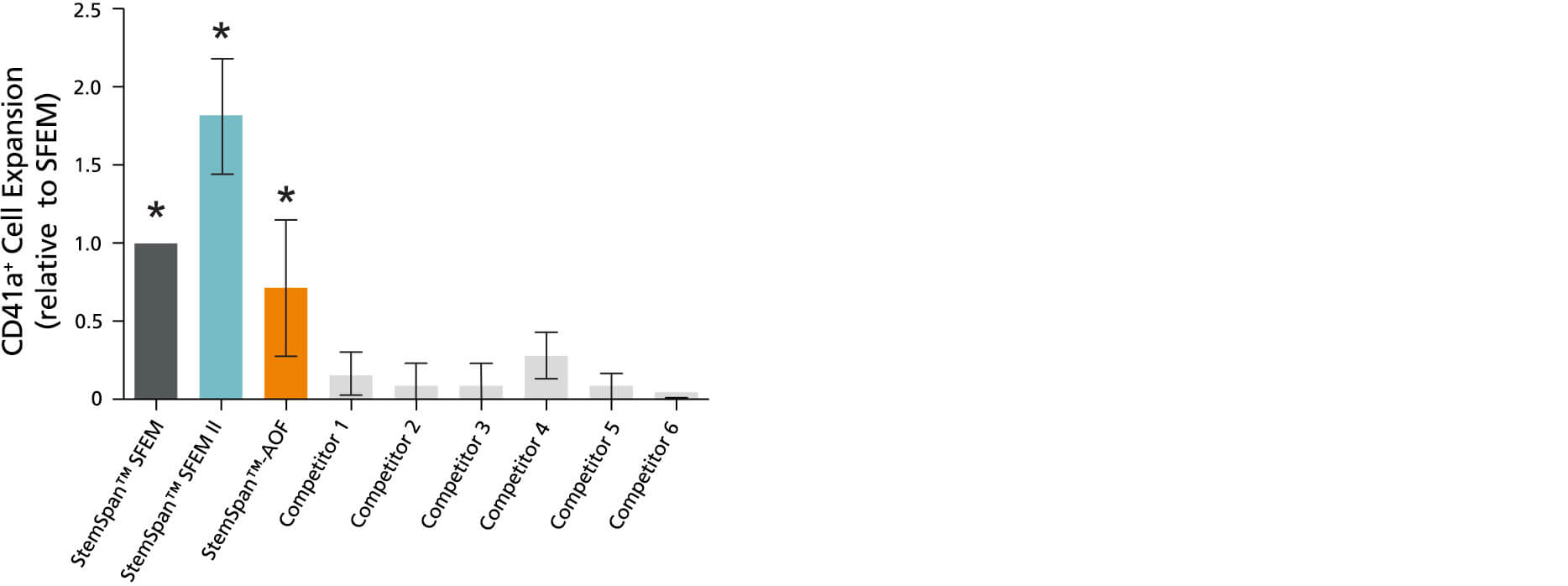
Figure 7. StemSpan™ SFEM II Serum-Free Expansion Medium Containing Megakaryocyte Expansion Supplement Supports Greater Expansion of Megakaryocytes Than Other Media Tested
The numbers of megakaryocytes, normalized relative to the values obtained in StemSpan™ SFEM medium (dark gray bar), obtained after culturing purified CD34+ CB cells for 14 days in StemSpan™ SFEM, SFEM II (gold bar) and AOF (orange bar), and six media from other commercial suppliers (light gray bars; Competitor 1-6, which included, in random order, StemLine II (Sigma), HPGM (Lonza), HP01 (Macopharma), SCGM (Cellgenix), StemPro34 (Life Technologies) and X-Vivo-15 (Lonza)). All media were supplemented with StemSpan™ Megakaryocyte Expansion Supplement (Catalog #02696). Vertical lines indicate 95% confidence limits, the range within which 95% of results fall. The numbers of cells produced in the StemSpan™ media were significantly higher than in the other media (*p<0.01 paired t-test, n=6).
Note: Data for StemSpan™-AOF shown were generated with the original phenol red-containing version (Catalog #09855). However internal testing showed that the performance of the new phenol red-free, cGMP-manufactured version of StemSpan™-AOF(Catalog #100-0130) was comparable.
Table 1. Production of Myeloid Cells from Human CB CD34+ Cells Cultured in SFEM II Containing Myeloid Expansion Supplement or Myeloid Expansion Supplement II
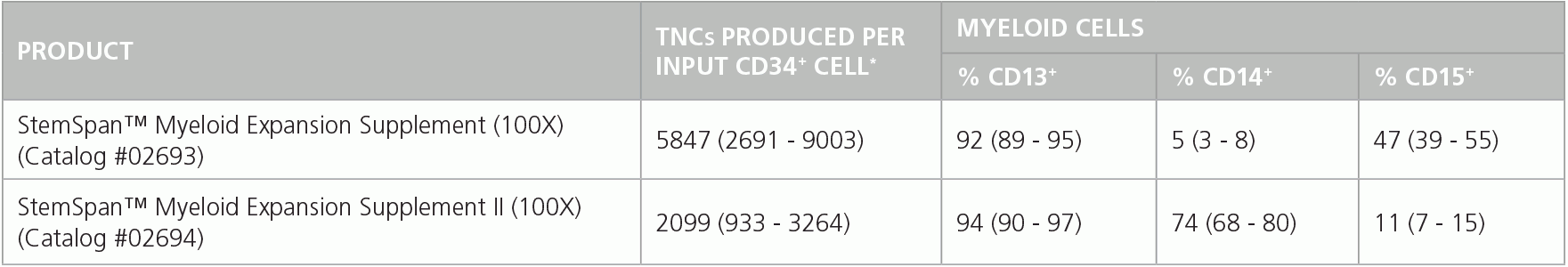
Shown are numbers of total nucleated cells (TNCs) produced per input human CB-derived CD34+ cell and percentages of cells positive for myeloid markers CD13, CD14 and CD15 after 14 days of culture in SFEM II containing Myeloid Expansion Supplement (n = 14) or Myeloid Expansion Supplement II (n = 16). *95% confidence limits (CL); the range within which 95% of results typically fall.
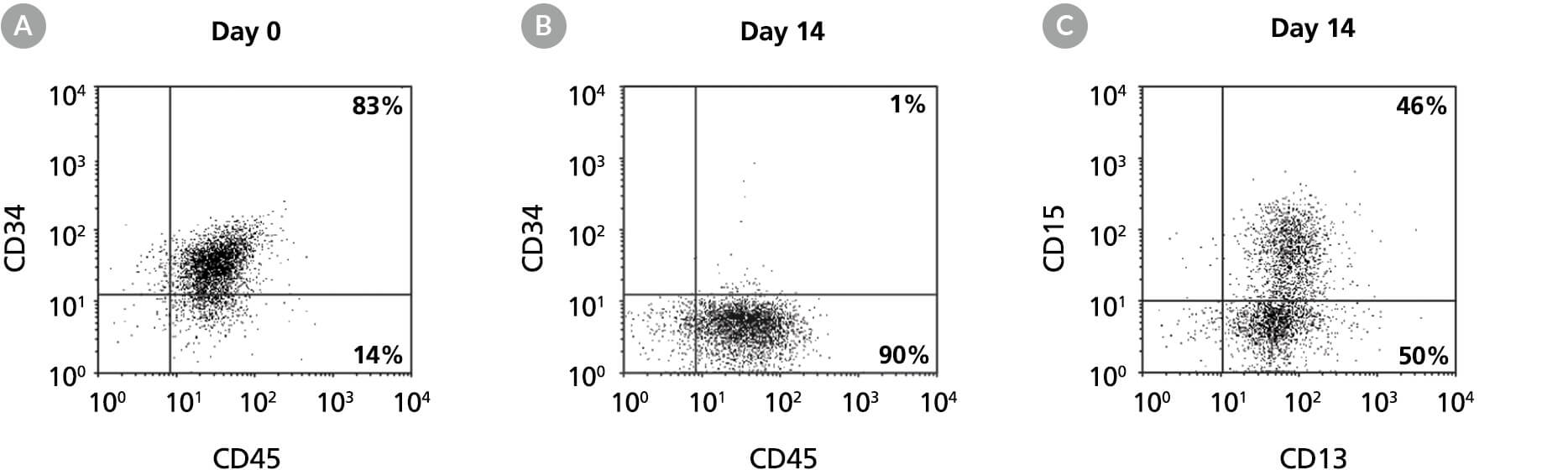
Figure 8. Production of CD13+ and CD15+ Myeloid Cells by the Expansion and Lineage-Specific Differentiation of Human BM-Derived Cells Cultured in StemSpan™ SFEM II Containing Myeloid Expansion Supplement
Flow cytometry dot plots for 14-day cultures of human BM-derived CD34+ cells in StemSpan™ SFEM II containing Myeloid Expansion Supplement showing expression of the HSPC marker CD34, and pan-hematopoietic marker CD45 (A) before and (B) after 14 days of culture, and expression of myeloid markers CD13 and CD15 (C) after 14 days of culture. The frequency of CD34+ cells declined from 83% before culture to ~1% after 14 days. In parallel, myeloid CD13+CD15+ cells gradually accumulated from <10% on day 0 to 46% by day 14. The frequency of CD14+ cells after culture was low (typically <10%).
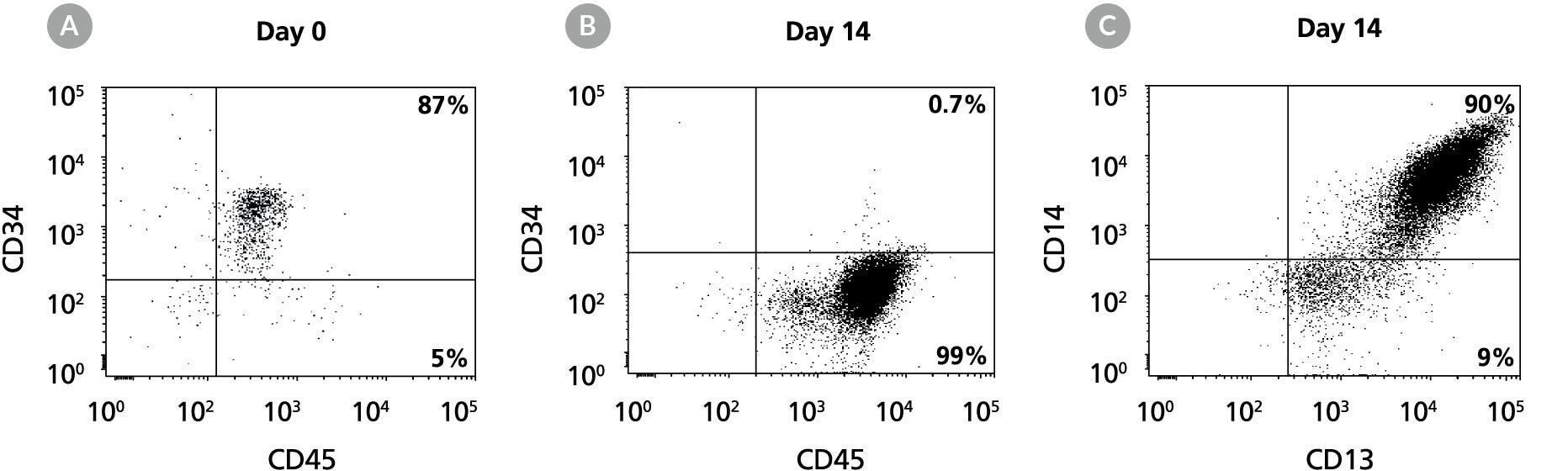
Figure 9. Production of CD14+ Monocytes by the Expansion and Lineage-Specific Differentiation of Human CB CD34+ Cells Cultured in StemSpan™ SFEM II Containing Myeloid Expansion Supplement II
Flow cytometry dot plots for 14-day cultures of human CB CD34+ cells in StemSpan™ SFEM II containing Myeloid Expansion Supplement II. The plots show expression of CD34 and CD45 (A) before and (B) after culture, and (C) expression of CD13 and CD14 on the expanded cells. The frequency of CD34+ cells declined from 87% before culture to less than 1% after 14 days. CD14+ monocytes increased from < 5% on day 0 to 90% by day 14.
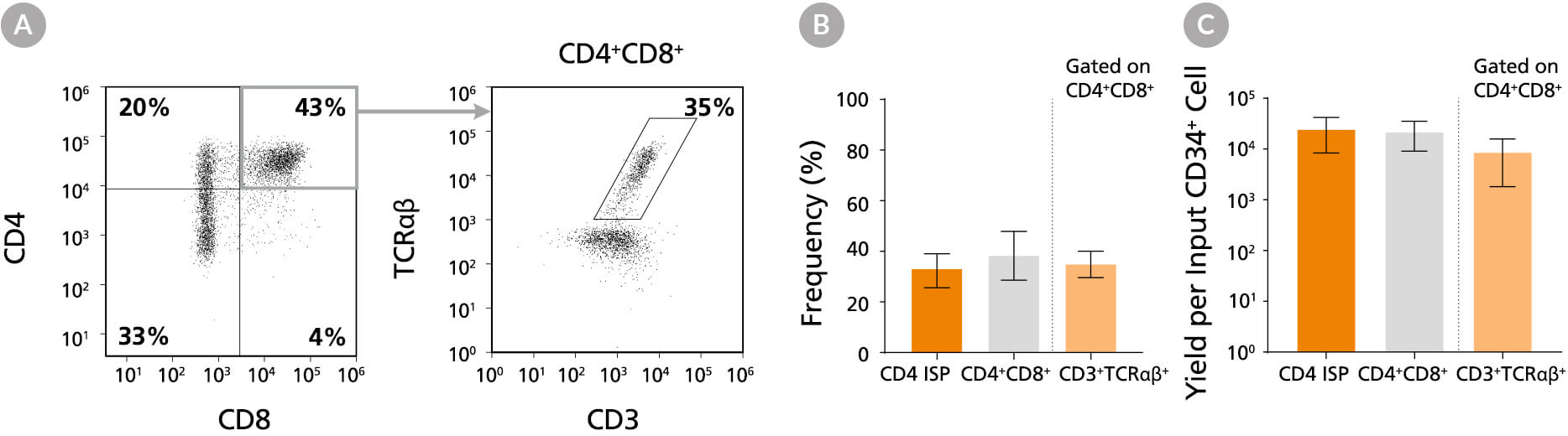
Figure 10. Frequency and Yield of CD4 ISP and CD4+CD8+ DP Cells After 42 Days of Culture
CB-derived CD34+ cells (freshly isolated or frozen) were cultured with the StemSpan™ T Cell Generation Kit (Catalog #09940) for 42 days and (A) analysed by flow cytometry for the expression of CD4, CD8, CD3 and TCRαβ. The (B) frequency and (C) yield of CD4 ISP, double-positive (CD4+CD8+) and CD3+TCRαβ+-expressing double-positive cells (CD4+CD8+CD3+TCRαβ+) are shown. On average, 38% of the total viable population were DP (CD4++), of which 35% co-expressed CD3 and TCRαβ. The yields of total DP cells and CD3+TCRαβ+ DP cells per input CD34+ cell were ~23,000 and ~9,000, respectively. Shown are means with 95% confidence intervals (n = 31).
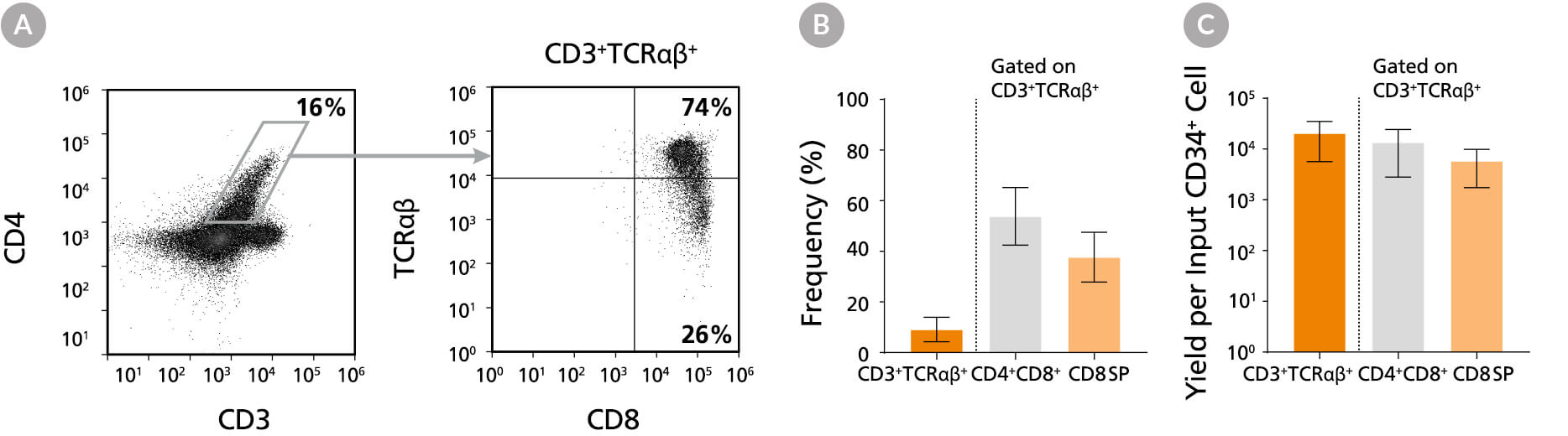
Figure 11. Frequency and Yield of CD8 SP T Cells After 49 Days of Culture
DP cells were further matured into CD8 SP T cells by culturing for an additional 7 days in StemSpan™ SFEM II with T Cell Progenitor Maturation Supplement (Catalog #09930), IL-15 (Catalog #78031) and ImmunoCult™ CD3/CD28/CD2 T Cell Activator (Catalog #10970) on coated plates. On day 49, cells were (A) analyzed by flow cytometry for the expression of CD3, TCRαβ, CD4 and CD8. The (B) frequency and yield of CD3+TCRαβ+-expressing cells and their subsets are shown. On average, 54% of the CD3+TCRαβ+ cells were DP (CD4+CD8+) and 38% were CD8 SP (CD4-CD8+). The average yield of CD8 SP T cells per input CD34+ cell was ~6,000. CD3+TCRαβ+ CD4 SP (CD4+CD8-) T cells were detected at very low frequencies (data not shown). Shown are means with 95% confidence intervals (n = 12).
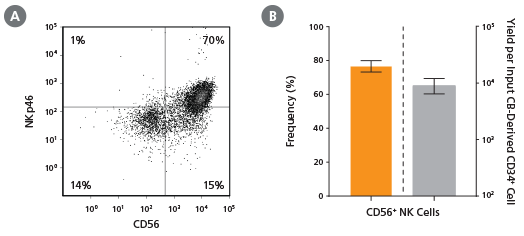
Figure 12. Frequency and Yield of CD56+ NK Cells After 28 Days of Culture
CB-derived CD34+ cells (freshly isolated or frozen) were cultured with the StemSpan™ T Cell Generation Kit (Catalog #09940) for 42 days and (A) analysed by flow cytometry for the expression of CD4, CD8, CD3 and TCRαβ. The (B) frequency and (C) yield of CD4 ISP, double-positive (CD4+CD8+) and CD3+TCRαβ+-expressing double-positive cells (CD4+CD8+CD3+TCRαβ+) are shown. On average, 38% of the total viable population were DP (CD4++), of which 35% co-expressed CD3 and TCRαβ. The yields of total DP cells and CD3+TCRαβ+ DP cells per input CD34+ cell were ~23,000 and ~9,000, respectively. Shown are means with 95% confidence intervals (n = 31).
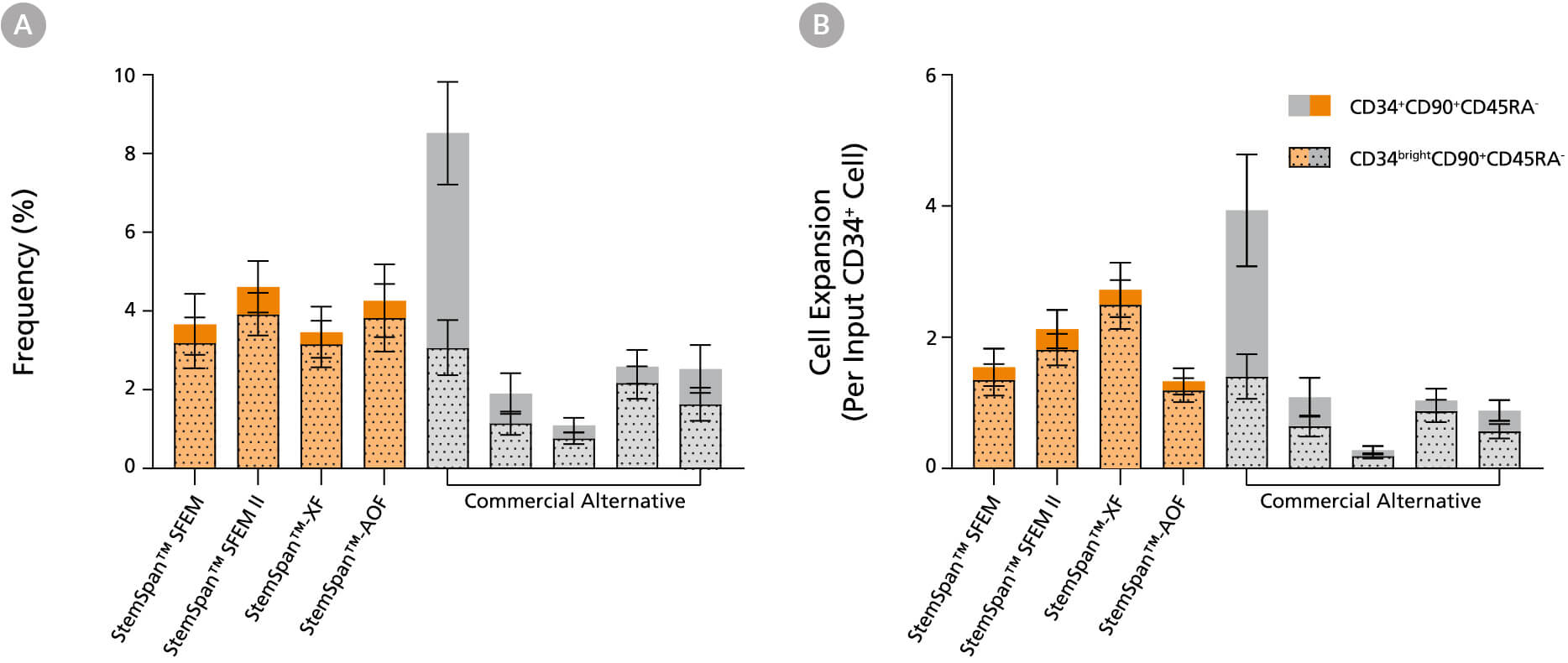
Figure 13. StemSpan™ Media Support Equal or Greater Expansion of Primitive Human CD34brightCD90+CD45RA- Cells Than Other Commercial Media
Purified CB-derived CD34+ cells were cultured for 7 days in StemSpan™ media (SFEM, SFEM II, AOF, and XF, orange bars), and in five media from other suppliers (Commercial Alternative, grey bars). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM171*. The (A) frequency and (B) cell expansion of viable CD34+CD90+CD45RA- (solid) and CD34brightCD90+CD45RA- (dotted overlay) cells in culture were analyzed by flow cytometry as described in Figure 1. Compared to the competitor media tested, StemSpan™ media showed similar or significantly higher expansion of CD34brightCD90+CD45RA- cells (P < 0.05 when comparing StemSpan™ SFEM II and XF to five media from other suppliers, calculated using one-way ANOVA followed by Dunnett’s post hoc test). The CD34brightCD90+CD45RA- cell population is highly enriched for functional stem/progenitor cells. Data shown are mean ± SEM (n = 8).
*Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1μM. For more information including data comparing UM171 and UM729, see Fares et al. 2014.
Note: Data for StemSpan™-AOF shown were generated with the original phenol red-containing version (Catalog #09855). However internal testing showed that the performance of the new phenol red-free, cGMP-manufactured version of StemSpan™-AOF(Catalog #100-0130) was comparable.
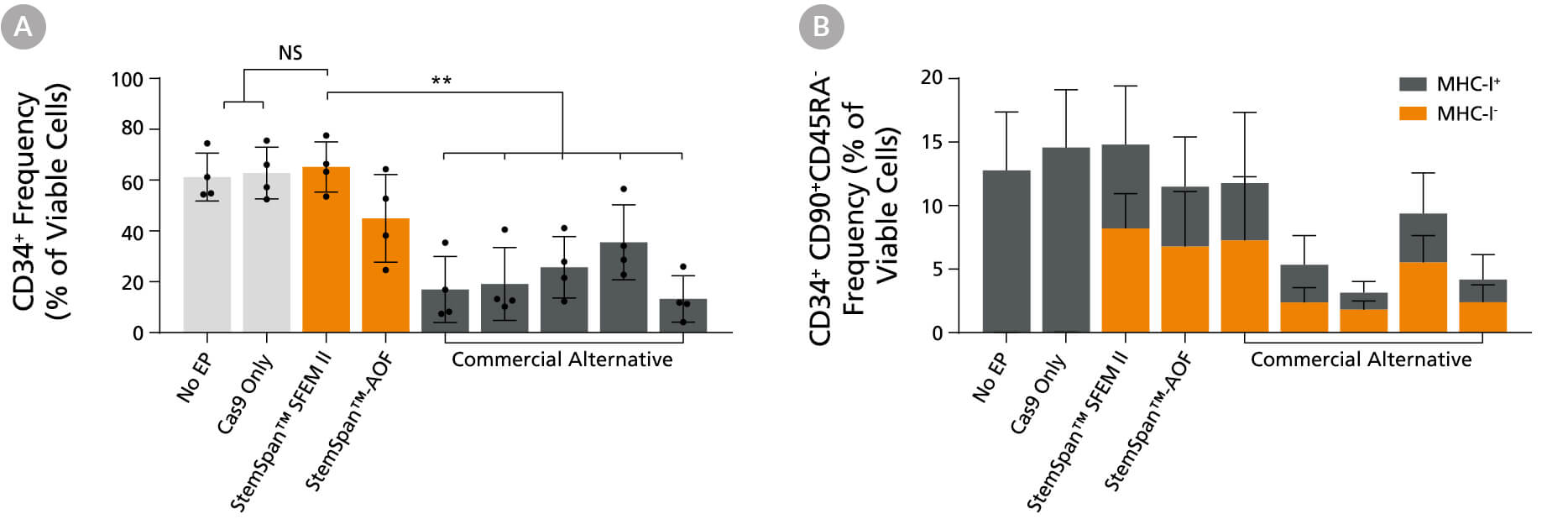
Figure 14. StemSpan™ Media Support Better CD34+ and Primitive CD34+CD90+CD45RA- HSPC Expansion in a Genome Editing Application Compared with Alternative Commercial Media
Purified CB-derived CD34+ cells were cultured for 2 days in select StemSpan™ media (StemSpan™ SFEM II or StemSpan™-AOF, orange bars), or five xeno-free media formulations from other suppliers (gray bars). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM171*. Cells were then electroporated using Arcitect™ CRISPR-Cas9 RNP complexes containing crRNA:tracrRNA targeting beta-2-microglobulin (B2M), and cultured for an additional 4 days in the same conditions. Knockout efficiency as measured by staining for MHC-I and analyzing by flow cytometry, was similar in all media tested, ~70-80%. (A) The percentage of CD34+ cells and (B) CD34+CD90+CD45RA-cells were quantified by flow cytometry 4 days post-electroporation. Data shown are mean + SD (n = 4 donors; **P < 0.01).
*Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1 μM. For more information including data comparing UM171 and UM729, see Fares et al., 2014.
Note: Data for StemSpan™-AOF shown were generated with the original phenol red-containing version (Catalog #09855). However internal testing showed that the performance of the new phenol red-free, cGMP-manufactured version of StemSpan™-AOF(Catalog #100-0130) was comparable.

Figure 15. Colony-Forming Potential of CD34+ CML Cells is Maintained During Culture
CML cells were assayed in colony assays using MethoCult™ H4435 Enriched medium directly after CD34+ cell isolation (day 0) or after 7 or 14 days of expansion without or with UM171 (as described in Figure 2). After 14 days of culture in StemSpan™ SFEM II with CD34+ expansion supplement (Exp) with or without UM171, colonies were (A) imaged with STEMvision™ and counted manually from digital images. (B) CFU output expressed as the total number of colonies per original input CD34+ cell. Numbers above each of the individual bars indicate the proportion of BCR-ABL positive colonies, measured by qRT-PCR on individual plucked colonies across 6 different samples (8-12 colonies were plucked for each sample per condition). SFEM II supplemented with CD34+ Expansion Supplement (Exp) supports the expansion of colony-forming progenitor cells in culture. UM171 further promotes colony forming progenitor cell output (~3.5-fold expansion at day 7 and ~8-fold at day 14). Single-colony qRT-PCR analysis revealed that colonies generated from samples on day 0, and colonies generated from cells expanded for 7 and 14 days, were predominantly BCR-ABL+ but also that normal BCR-ABL- progenitor cells were present at low frequencies. Data shown are mean ± SEM (n = 6). P-values were calculated using a two-tailed paired Student’s t-test (*P < 0.05).

Figure 16. Colony-Forming Potential of CD34+ AML Cells is Maintained During Culture
AML cells, after CD34+ cell isolation (day 0) or after 7 or 14 days of expansion without or with UM171 (as described in Figure 3), were plated in colony assays with MethoCult™ H4435 Enriched medium. After 14 days of incubation, colonies were (A) imaged with STEMvision™ and counted manually from digital images. (B) CFU output expressed as the total number of colonies per original input CD34+ cell. SFEM II supplemented with CD34+ Expansion Supplement (Exp) supports the expansion of colony-forming progenitor cells in culture. Addition of UM171 further promotes colony-forming progenitor cell output (~3-fold expansion at day 7 and ~4-fold at day 14). Data shown are mean ± SEM (n = 6). P-values were calculated using a two-tailed paired Student’s t-test (*P < 0.05).
Scientific Resources
Key Applications
Generation of Mature Blood Cells in Vitro
Leberbauer et al. (2005) Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood 105(1)
SFEM II
Huijskens et al. (2014) Technical advance: ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J Leukoc Biol 96(6)
CC100
Kumkhaek et al. (2013) MASL1 induces erythroid differentiation in human erythropoietin-dependent CD34+ cells through the Raf/MEK/ERK pathway. Blood 121(16)
CC110
Gaikwad et al. (2007) In Vitro Expansion of Erythroid Progenitors from Polycythemia Vera Patients Leads to Decrease in JAK2V617F Allele. Exp Hema 35(4)
Ex Vivo Expansion of HSPCs for Rapid/Sustained Hematopoietic Recovery Post Transplantation
Delaney et al. (2010) Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med 16(2)
Cutler et al. (2013) Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood 122(17)
Generating Target Cells for Reprogramming to Make Induced Pluripotent Stem Cells
Ohmine et al. (2011) Induced pluripotent stem cells from GMP-grade hematopoietic progenitor cells and mononuclear myeloid cells. Stem Cell Res and Therapy 2(6)
Gene Transfer into HSPCs
Lechman et al. (2012) Attenuation of miR-126 Activity Expands HSC In Vivo without Exhaustion. Cell Stem Cell 11(6)
SFEM II
Buechele C et al. (2015) MLL leukemia induction by genome editing of human CD34+ hematopoietic cells. Blood. Epub ahead of print