Frequently Asked Questions on PneumaCult™ Alveolar Organoid Media
Find answers to frequently asked questions (FAQs) about culturing human alveolar cells using PneumaCult™ Alveolar Organoid Media.
Can't find the answer you are looking for? Fill out our short form, and our pulmonary specialists will get back to you.
General Information
Can I use PneumaCult™ Alveolar Organoid Media to expand and differentiate alveolar cells?
Yes, PneumaCult™ Alveolar Organoid Expansion Medium was developed for the passaging and long-term expansion of human alveolar epithelial type II (ATII) cells as organoids. The ATII organoids can also be further differentiated into alveolar epithelial type I (ATI) cells for downstream applications, using PneumaCult™ Alveolar Organoid Differentiation Medium.
Unsure which culture media to choose? Try our interactive product finder to find the right media for your pulmonary research >
Learn more about PneumaCult™ Culture Media for Airway Epithelial Cells and Pulmonary Organoids.
What samples can be used with PneumaCult™ Alveolar Organoid Media?
PneumaCult™Alveolar Organoid Media is compatible with primary isolated fresh biopsy lung tissue or cryopreserved ATII cells, as well as high-quality commercially available alveolar epithelial cell sources with adequate concentrations of HT2-280+ cells (>25%). For example, Adult Human Alveolar Epithelial Cells (ScienCell Catalog #3240) work well with our media.
Can the PneumaCult™ Alveolar Organoid Media be used for the expansion of human pluripotent stem cells (hPSC)-derived alveolar epithelial type II (ATII) cells?
Is PneumaCult™ Alveolar Organoid Media serum-free?
PneumaCult™ Alveolar Organoid Expansion Medium is a three-component, serum-free medium. However, the two-component PneumaCult™ Alveolar Organoid Differentiation Medium contains serum.
Materials and Plate Preparation
What tissue-culture plates should I use?
We recommend using Non-Treated, Flat-Bottom Plates. We have found that non-tissue culture-treated plates minimize 2D attachment, and the form of the dome is improved compared to tissue culture-treated plates.
When using non-tissue culture-treated plates, the surface tension properties of this plastic may result in taller, rounder domes, with less spread and flattening. Ensure the domes are incubated at 37°C for 40 to 50 mins to gel properly and use Matrigel® with protein concentrations ≥ 8.0 mg/mL. To reduce the dome height, perform a swirl motion while setting down the domes to enlarge the foot of the dome and reduce the dome height.
Can other plate formats be used (e.g. 96 wells)?
Yes, other plate sizes can be used during the alveolar organoid expansion phase using PneumaCult™Alveolar Organoid Expansion Medium. The cultureware should be non-tissue culture treated to prevent cells from attaching to the bottom of the plate. For a 96-well plate format, we suggest seeding between 200-1600 cells per 4 μL dome for the expansion phase. Seeding densities for differentiation into alveolar epithelial type I (ATI) cells in a 96-well plate requires further optimization by the user.
What matrix should I use?
PneumaCult™ Alveolar Organoid Media was optimized using Corning® Matrigel® Growth Factor Reduced (GFR) Basement Membrane Matrix, Phenol Red-free (Corning® Catalog #35623). We have not qualified a Corning® Matrigel® alternative to be used with our PneumaCult™ Alveolar Organoid Media, but given the similarities between Corning's Matrigel® and Trevigen's Cultrex® 3D Basement Membrane Matrix, GFR (Trevigen Catalog #3445-001-01) it is likely to work. We suggest the matrix contains a minimum protein concentration of 8 mg/mL.
Sample Preparation
Can I use freshly isolated tissue from a biopsy or lung tissue?
PneumaCult™Alveolar Organoid Media can be used with freshly isolated tissue. We recommend using HT2-280+ flow-sorted freshly isolated ATII cells with adequate concentrations of HT2-280+ cells (>25%). See Table 1 in Question 2 of the “Characterization” section for antibody recommendations.
Do you have a protocol for processing fresh tissue samples?
We suggest using fresh tissue over frozen tissue, when possible. We do not have a validated protocol for tissue dissociation in-house. However, for a fresh tissue digestion protocol suggestion prior to HT2-280 cell sorting, please refer to Katsura et al. 2020.
This paper outlines the human lung tissue dissociation protocol and the isolation of human epithelial type II (ATII) cells using a FACS-based protocol.
- Katsura et al. (2020) Human lung stem cell-based alveolospheres provide insights into sars-cov-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell 3;27(6):890-904.
Do you have any recommendations for vendors of ATII cells that can be used to establish organoid cultures?
Adult Human Alveolar Epithelial Cells (ScienCell Catalog #3240) have worked well to establish, expand, and differentiate alveolar organoids using PneumaCult™ Alveolar Organoid Media.
Does PneumaCult™ Alveolar Organoid Media support the culture of murine alveolar cells?
We have not tested the PneumaCult™ Alveolar Organoid Kit with mouse epithelial type II (ATII) cells in-house. For mouse alveolar epithelial cell culture, please refer to Katsura et al. 2020.
Explore this article citing the culture of murine alveolar epithelial type II (ATII) cells:
- Katsura et al. (2020) Human lung stem cell-based alveolospheres provide insights into sars-cov-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell 3;27(6):890-904.
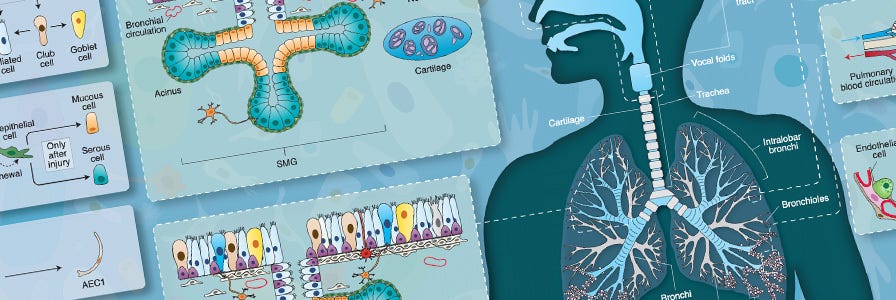
Wallchart: Cellular Organization and Biology of the Respiratory System
Get an overview of the different parts of the respiratory system, associated respiratory diseases, and airway cell culture model systems.
Setup and Culture
Do Matrigel® domes have to be 50 µL?
Dome sizes can be as small as 2 µL, as long as the cell density to Matrigel® remains at a consistent ratio of 4000 cells per 50 µL of the 1:1 Matrigel/PneumaCult™ Alveolar Organoid Expansion Medium mixture.
Can the organoids generated using the PneumaCult™ Alveolar Organoid Media be passaged?
Yes, alveolar expansion cultures (ATII cells) cultured in PneumaCult™ Alveolar Organoid Expansion Medium can be passaged as single cells. ATII cells serve as alveolar stem cells and can differentiate into alveolar epithelial type I (ATI) cells using the PneumaCult™ Alveolar Organoid Differentiation Medium. However, ATI cells are considered to be terminally differentiated and, therefore, not expandable in our medium.
See the passaging protocol in section 5.4 of the Technical Manual.
How many times can ATII cells be passaged?
For some donors, the cultures can last up to P12. However, we have seen that from passages 8 to 10; expansion cultures get exhausted, displaying less expansion and a decrease in organoid-forming efficiency (OFE). However, the potential of ATII cells to differentiate into ATI cells using the PneumaCult™ Alveolar Organoid Differentiation Medium after long-term passage remains unaffected.
What is the significance of organoid-forming efficiency (OFE)?
OFE is a measure of the culture’s stemness. It provides a measure of the number of organoids (e.g. ~200 organoids) per input cell (e.g. 4000 ATII cells). In a typical healthy expansion organoid culture, an OFE ≥ 5% is to be expected, which diminishes with culture passage. OFE is a measure of self-renewal and organogenic capacity of the seeded cells that can serve as a readout for applications such as drug or toxicity assays.
Please refer to section 7.3 of the Technical Manual to learn how to perform an OFE.
How many days can I keep the organoids in the differentiation phase?
The initiation phase lasts 7 - 14 days, after which cultures can be differentiated using PneumaCult™ Alveolar Organoid Differentiation Medium for a further 10 days. Beyond Day 10 in differentiation, organoid domes may start collapsing due to the accumulated number of proteases that are secreted by the cells, causing the matrix to break apart after prolonged culture.
How long does it take for alveolar organoids to fully differentiate?
PneumaCult™ Alveolar Organoid Differentiation Medium (AvOD) enables in-dome differentiation of ATII organoid cultures to ATI cells in as few as 7 to 10 days, with over 85% purity. Differentiated cells show decreased ATII marker expression and strong up-regulation of ATI cell markers (RAGE/AGER, HT1-56, and GPRC5a). Cultures are considered differentiated after 10 days in the differentiation medium (~10 days in PneumaCult™ Alveolar Organoid Expansion Medium (AvOE) Seeding and AvOE Medium + 10 days in AvOD = total culture for 20 days or 14 days in AvOEM + 10 days in AvOD = 24 days in culture). The differentiation phase will account for 10 days in total.
See Figure 1 in the PneumaCult™ Alveolar Organoid Media product page to view the detailed outline of the two-stage protocol.
My differentiated domes look like they are breaking apart; there are fragments and cells floating in the media and inside the domes. Is there an explanation for this?
This phenomenon is normal and expected, and does not affect the quality of the culture. Its occurrence is less frequent when using PneumaCult™ Alveolar Organoid Media compared to DIY medium. Differentiated cells begin to secrete proteases that start breaking apart the matrix and cell-cell contacts, causing the domes to fall apart, resulting in single or fragmented cells floating around (Figure 1).
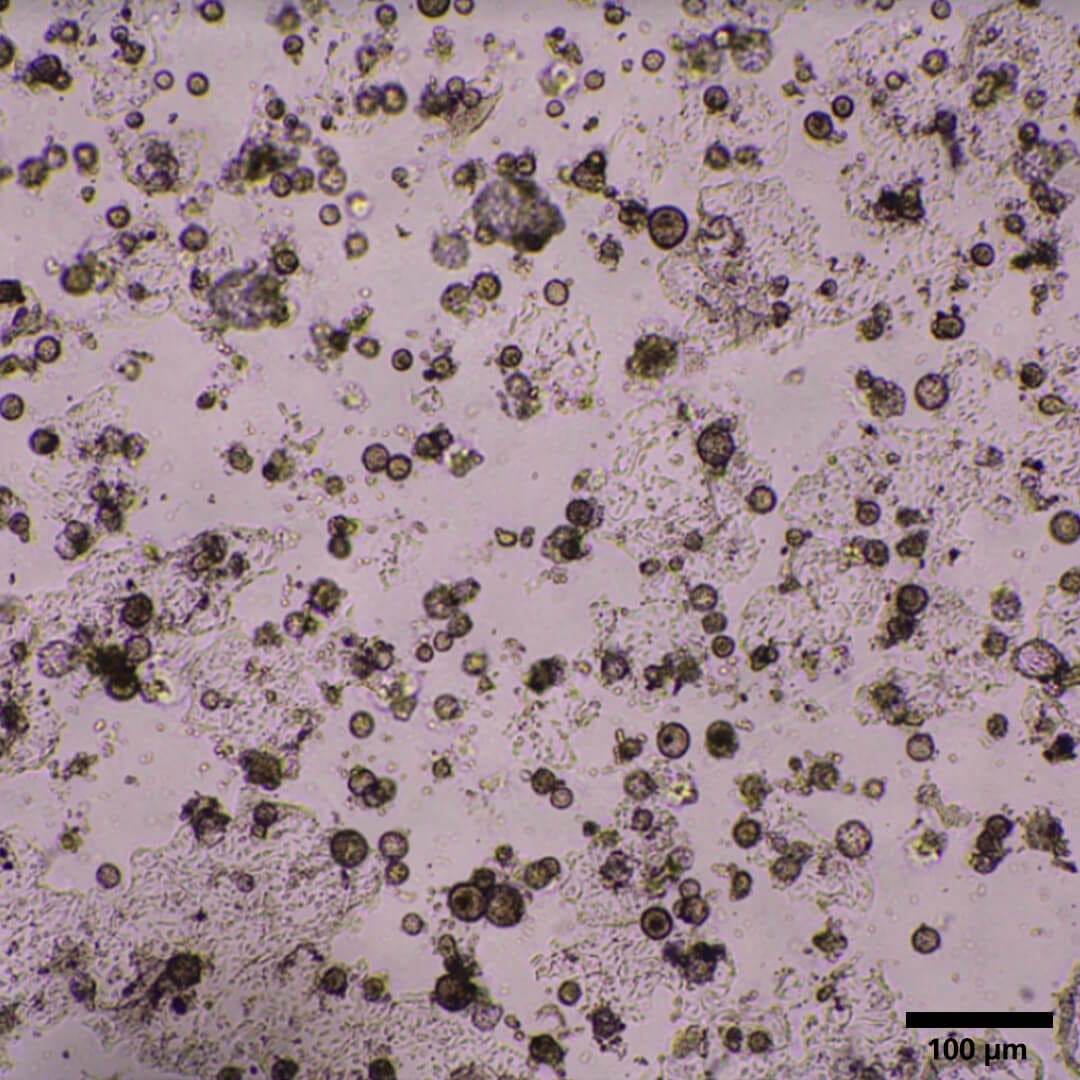
Figure 1. Brightfield Image of Differentiated Alveolar Organoids on Day 19.
On Day 8 of the differentiation stage, the alveolar organoids are seen with debris composed of single or fragmented cells as a result of the breakdown of the matrix. Magnification: 2X.
My cultures look dense in images, but the live cell counts are unusually low. What could be happening?
This is likely due to insufficient mixing after the addition of the Animal Component-Free Inhibition Solution. To avoid this, ensure the cell mixture is properly mixed by pipetting up and down at least five times after the inhibition solution is added. When working with larger numbers of wells, avoid dissociating more than 12 wells at a time to minimize over-incubation.
Is it expected that the differentiation potential drops with later passages?
We have differentiated alveolar organoids that have been expanded up to P10. These had a lower yield, but the organoid differentiation was up to 90%. In fact, earlier passages (P1 or P2) can sometimes get lower differentiation (~80%), possibly because these have mixed-in airway basal cells, which may lower the differentiation efficiency. However, after one or two passages, the basal cells should be eliminated.
Can Trypsin or Gentle Cell Dissociation Reagent (GCDR) be used for passaging instead of the Animal Component-Free Cell Dissociation Kit?
Trypsin-EDTA (0.05%) may work for passaging. However, the incubation and trypsin inhibition time would require optimization. For keeping serum-free media conditions, soybean trypsin inhibitors may be better than serum, though we have not tested the effects. GCDR would isolate the organoids as a whole.
Can viral infections be done in organoids in domes?
Yes, this is a feasible application. Katsura et al. 2020 used alveolar organoids as a model for SARS-CoV-2 infection. Here the authors grew alveolar organoids, added different molecules such as IFN, and then infected cultures with SARS-CoV-2 to see how the virus modulates the infection. We have not tested this in-house; however, our kit could be used for similar applications.
Explore this article citing the culture of murine alveolar epithelial type II (ATII) cells:
- Katsura et al. (2020) Human lung stem cell-based alveolospheres provide insights into sars-cov-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell 3;27(6):890-904.
For full protocol details, please refer to the Technical Manual under the Protocols and Documentation tab on the PneumaCult™ Alveolar Organoid Media product page.
Cryopreservation
Can alveolar organoids expanded in PneumaCult™Alveolar Organoid Expansion Medium (AvOEM) be cryopreserved? Do you have any recommendations for freezing medium?
Yes, alveolar organoids can be single-cell dissociated using the ACF Enzymatic Dissociation Kit and cryopreserved for biobanking during the expansion phase. We recommend freezing them down at 0.5 - 1 x 106 cells/mL using CryoStor® CS10. The volume of CS10 and the cell concentration can be scaled down to 200 µL. See Section 5.4 of the Technical Manual for the cryopreservation protocol and Section 5.2 for the thawing protocol.
Characterization
Do you have a protocol for RNA isolation of alveolar organoids?
The technical protocol How to Process Epithelial Organoids and Organoid-Derived Epithelial Monolayers for RNA Isolation describes a method for processing epithelial organoids for RNA extraction.
Do you have a protocol for performing immunocytochemistry (ICC) of alveolar organoids? Do you have any recommendations for antibodies?
The technical protocol Performing Immunocytochemical Staining of Epithelial Organoids describes a detailed whole-mount ICC staining protocol for epithelial organoids. Table 1 below shows marker recommendations for alveolar organoid characterization.
Table 1. Marker Recommendations for Alveolar Organoid Characterization using ICC
| Antibody | Catalog # | |
|---|---|---|
| Alveolar epithelial cells Type I (ATI) markers: | RAGE/AGER (Goat IgG) | R&D Systems AF1145 |
| GPRC5a (Rabbit IgG) | Sigma HPA007928 | |
| Alveolar epithelial cells Type II (ATII) markers * | Pro-SPC (Rabbit IgG) | Sigma AB3786 |
| HT2-280 (Mouse IgM) | Terrace Bio TB-27aHT2-280 | |
| General epithelial cell markers * | ACE2 (Rabbit IgG) | Abcam ab15348 |
| EpCAM/TROP-1 (Goat IgG) | R&D Systems AF960 |
* Not for mouse samples.
Compare the marker expression of ATI and ATII cells cultured in PneumaCult™ Alveolar Organoid Expansion Medium and PneumaCult™ Alveolar Organoid Differentiation Medium in Figures 5 and 6 on the product page.
Do you have a protocol on how to perform flow cytometry of alveolar organoids?
The Technical Manual, Section 7.0, outlines a protocol for immunostaining alveolar organoid cells for flow cytometry and the flow cytometry gating strategy.
My differentiated organoids still express ATII markers. Is this normal?
Yes, we have found some donors may retain their ATII markers more than others after differentiation. They will express ATI markers; nonetheless, there should be a drop in the intensity or frequency of ATII markers after differentiation.
Applications
Can ATII cells be cultured as a monolayer?
Yes, alveolar organoids generated using PneumaCult™ Alveolar Organoid Expansion Medium (AvOE), can be seeded as single cells into organoid cultures for long-term expansion across multiple passages. On Day 10 of the expansion culture, ATII cell-containing organoids can be seeded into cell culture inserts using PneumaCult™ AvOE Seeding and Expansion Media.
Please refer to the technical protocol How to Generate Alveolar Monolayers from ATII Organoids for detailed instructions.
Related Resources
References
- Katsura et al. (2020) Human lung stem cell-based alveolospheres provide insights into sars-cov-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell 27(6):890-904
- Kathiriya et al.(2020) Human alveolar type 2 epithelium transdifferentiates into metaplastic KRT5+ basal cells. Nature Cell Biology 24:10–23.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration