How to Generate Alveolar Monolayers from ATII Organoids
Alveolar type II (ATII) cells play a crucial role in maintaining homeostasis in the alveolus and as progenitors of alveolar type I (ATI) cells. Studying ATII cells in vitro is challenging due to their tendency to spontaneously differentiate into ATI cells. Therefore, a reproducible culture system that can prolong the ATII phenotype is valuable for gathering more insights on ATII cell function in co-cultures, disease modeling, and in drug screening and discovery.
Using PneumaCult™ Alveolar Organoid Expansion (AvOE) Medium, cryopreserved or freshly isolated human ATII cells can be seeded as single cells into organoid cultures for long-term expansion across multiple passages. On day 10 of expansion culture, ATII cell-containing organoids can be differentiated into ATI cells using PneumaCult™ Alveolar Organoid Differentiation (AvOD) Medium or seeded into cell culture inserts. This protocol describes the generation and analysis of alveolar monolayers from expansion-phase ATII organoids to facilitate air-liquid-interface (ALI) culture and enable easy access to the apical surface.
For complete instructions, use this document in coordination with the Generation of Human Alveolar Organoids Using PneumaCult™ Alveolar Organoid Media (Document #10000012979) technical manual.

Figure 1. Generating Alveolar Type 2 Cultures at the Air-Liquid Interface.
The generation of alveolar type 2 (ATII) cultures at the air-liquid interface (ALI) on cell culture inserts is a two-stage protocol. (A) In the first stage, primary isolated or cryopreserved human ATII single cells are seeded in PneumaCult™ Alveolar Organoid Expansion (AvOE) Medium as organoid cultures for 10 days. Prior to seeding on cell culture inserts, the inserts are coated with a combination of 2 extracellular matrices (ECMs). (B) Single cells are then harvested from the expanded ATII organoids and seeded into the ECM-coated cell culture inserts before being expanded for 3 - 4 days in PneumaCult™ Alveolar Organoid Seeding Medium. (C) After confluency is reached, the cells may be airlifted for ALI culture and (D) collected for assays on Day 7 or (E) cultured for up to an additional 10 days.
Materials
- PneumaCult™ Alveolar Organoid Expansion Medium (Catalog #100-0847)
- Heparin Solution (Catalog #07980)
- Animal Component-Free Cell Dissociation Kit (Catalog #05426)
- CorningⓇ MatrigelⓇ Growth Factor Reduced (GFR) Basement Membrane Matrix, Phenol Red-free (Corning Catalog #356231)
- 24-well non-tissue culture-treated plates (e.g. Catalog #100-0097)
- CellAdhere™ Buffer or PBS with Ca2+ and Mg2+ (e.g. Catalog #07183)
- Type III Collagen Solution (Advanced BioMatrix Catalog #5021)
Media Preparation
Preparation of Complete PneumaCult™ Alveolar Organoid Expansion (AvOE) Medium
Use sterile technique to prepare complete PneumaCult™ AvOE Medium. The following example is for preparing 100 mL of complete medium. If preparing other volumes, adjust accordingly.
- Thaw PneumaCult™ Alveolar Organoid Expansion 10X Supplement at room temperature (15 - 25°C) (~1 hour). Mix thoroughly, then store on ice.
- Aliquot 89.75 mL of PneumaCult™ Alveolar Organoid Expansion Basal Medium into a sterile container.
Note: If antibiotics are required, reduce basal medium volume to accommodate antibiotic volume.
- Add 10 mL of PneumaCult™ Alveolar Organoid Expansion 10X Supplement.
Note: If not used immediately, aliquot supplement and store at -20°C. After thawing aliquots, use immediately; do not refreeze. Do not exceed the expiry date as indicated on the label. Alternatively, store supplement at 2 - 8°C for up to 1 week.
- Add 250 µL Heparin Solution. Mix thoroughly, avoiding generating bubbles.
- Optional: Add antibiotic(s)/antimycotic(s) as desired.
Note: If not used immediately, store the complete medium at 2 - 8°C for up to 6 weeks. Do not exceed the expiry date of the individual components.
Preparation of PneumaCult™ AvOE Seeding Medium
Use sterile technique to prepare PneumaCult™ AvOE Seeding Medium. The following example is for preparing 10 mL of seeding medium. If preparing other volumes, adjust accordingly.
- Thaw PneumaCult™ Alveolar Organoid Expansion 100X Passage Supplement at room temperature (~30 minutes). Mix thoroughly, then store on ice.
Note: If not used immediately, aliquot supplement and store at -20°C. After thawing aliquots, use immediately; do not refreeze. Do not exceed the expiry date as indicated on the label. Alternatively, store supplement at 2 - 8°C for up to 1 week.
- Add 9.9 mL of complete PneumaCult™ AvOE Medium to a new sterile container.
- Add 100 µL of PneumaCult™ Alveolar Organoid Expansion 100X Passage Supplement to the aliquoted medium. Mix thoroughly. Use immediately.
For information on how to expand the cells as organoids, follow the Generation of Human Alveolar Organoids Technical Manual.
Protocol
The following protocol is for a 24-well cell culture insert plate. For other cultureware, adjust volumes accordingly.
Note:
- Cells may be maintained in a submerged culture (no air lifting).
- Cells may be maintained at ALI for 7 - 14 days, depending on the cell quality and donor. Over time, the cells will begin to lose ATII marker expression.
- After 7 or 14 days, cultures will start to form multiple layers or holes in the cultures, depending on the cell quality and donor. Holes and multiple layers may be due to continued expansion of the cells in AvOE Medium in 2D, and may not be desirable if a continuous monolayer is required. Submerged cultures are more likely to form multiple layers and holes may form, compared to cultures at ALI.
Coating Cell Culture Inserts
- Prepare Collagen III solution by diluting 1 mg/mL stock to 100 µg/mL in 0.01 M HCl.
- Add 100 µL of 100µg/mL Collagen III solution to each cell culture insert.
- Leave at room temperature (19 - 23°C) for 1.5 - 3 hours.
- Wash (200 µL apical, 500 µL basolateral) 3x with PBS.
- Prepare Biolaminin 332 solution by diluting 100 µg/mL stock to 10 µg/mL in CellAdhere Buffer or PBS with Ca2+ and Mg2+.
- Add 100 µL of the 10 µg/mL Biolaminin 332 solution to each cell culture insert, layered on top of the Collagen III coating.
- Incubate overnight at 4°C.
- If not using immediately, wash with CellAdhere buffer or PBS with Ca2+ and Mg2+ and store at 4°C in a sufficient volume of CellAdhere buffer to cover the insert, or PBS Ca2+ and Mg2+, sealed in parafilm to prevent evaporation. Plates can be stored for up to 4 weeks.
Passage Alveolar Organoids and Seed Alveolar Cells Into Coated Inserts
- Culture ATII cells as described in PneumaCult™ AvOE Medium Technical Manual.
- Briefly, seed 4000 ATII cells per dome in a 50% Matrigel, 50 µL dome.
- Culture Matrigel domes using PneumaCult™ AvOE Seeding Medium for the first 2 - 3 days, then change medium to PneumaCult™ AvOE Medium.
- Culture for an additional 7 - 14 days by changing PneumaCult™ AvOE Medium every 3 - 4 days.
Note: Approximately one dome of alveolar organoids in expansion-phase is needed per cell culture insert to be seeded. - Passage cells from 3D dome as described in PneumaCult™ AvOE Medium Technical Manual into single cells.
- Add 500 µL ACF Enzymatic Dissociation Solution per 50 µL Matrigel® in the well.
- Triturate by pipetting up and down 10 times forcefully and quickly (avoid bubble formation by stopping at the first stop of the pipette) to shear large Matrigel® aggregates.
- Incubate at 37°C for 10 minutes.
- Using a 1 mL pipette, triturate each well again, 5 - 10 times. Incubate at 37°C for 5 minutes.
- Triturate 5 times, then add an equal volume of ACF Enzyme Inhibition Solution. Mix by pipetting 3 - 5 times. Immediately transfer to sterile 15 mL conical or microcentrifuge tubes as volumes permit.
- Centrifuge at 400 x g for 5 minutes at 2 - 8°C.
- Aspirate supernatant, then resuspend cells in a minimum volume of 250 - 500 µL of room temperature complete PneumaCult™ AvOE Medium. Mix by pipetting 5 - 8 times.
- Perform a live cell count.
- Wash ECM-coated cell culture insert and add 500 µL PneumaCult™ AvOE Seeding Medium into the basolateral chamber of the cell culture flask and up to 200 µL into the apical chamber of the insert.
- Adjust cell concentration to 30,000 cells per 200 µL medium (150,000 per 1 mL).
- Seed 30,000 cells per cell culture insert.
Note: The remaining cells may be cryopreserved as described in the Alveolar Organoid Technical Manual.
Changing Medium and Air Lifting
Cells are confluent after approximately 3 - 4 days post seeding. Once cells have formed a confluent monolayer, alveolar cultures may be airlifted as follows:
- Carefully aspirate media from the apical side of the cell culture insert using manual pipetting. Be sure to not scratch or touch the cells.
- Remove media from the basolateral chamber and replace with 500 µL PneumaCult™ AvOE Medium.
- Monitor cells after air lifting, as medium may flow from the basolateral side to the apical side. This should not occur if cells are completely confluent, but may depend on the quality of the donor. Remove any medium from the apical side on subsequent days and monitor confluency. Cells can be cultured for 3 - 11 more days at the ALI. Change medium every 3 - 4 days.

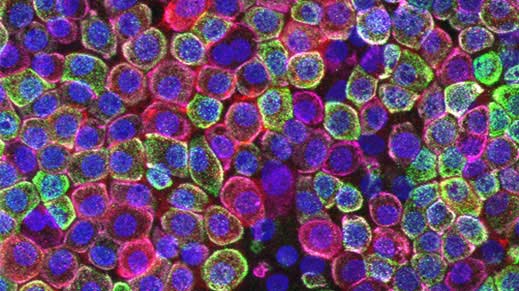
Figure 2. ATII Cells Show Optimal Marker Expression When Cultured for 7 Days As Submerged or ALI Cultures.
ATII cells from two separate donors were seeded into ECM-coated inserts and cultured to Day 7 or Day 14. Cultures were placed at ALI on day 4 and cultured for an additional (C, D) 3 or (G, H) 10 days. Cells were fixed on the membrane in 4% PFA and stained for the ATII-specific marker HT2-280 (green), as well as the ATI-specific markers RAGE (red) and GPRC5a (magenta) and counterstained with DAPI (blue). In both donors, cultures in (A, B) submerged and (C, D) ALI for 7 days express HT2-280 with some RAGE, and little GPRC5a. Additional growth for 7 days in (E, F) submerged cultures resulted in a decrease in HT2-280 and an increase in RAGE, indicating differentiation into ATI cells. (G, H) Growth for an additional 7 days at the ALI resulted in some decrease in HT2-280, but expression was more stable than in submerged culture conditions. This data suggests that the ideal culture conditions for ATII cells on cell culture inserts are for (C, D) 7 days, with 3 days at ALI; however these cultures can still be maintained for up to 14 days (10 days of ALI) with minimal loss of ATII markers. Note scale is the same for XY and ZX figures.
Immunostaining
Immunocytochemical staining of the alveolar cells on permeable inserts is performed as follows, as a modification of the Performing Immunocytochemical Staining of Epithelial Organoids Protocol.
Preparation of Buffers
- Prepare a 4% PFA Solution by combining the following:
- 10 mL of 16% PFA (e.g. Pierce™ 16% Formaldehyde (w/v), Methanol-Free, Thermo Scientific™ Catalog #28908)
- 30 mL PBS
Note: 4% PFA Solution may be aliquoted and stored at -20°C for 6 months. Avoid multiple freeze-thaws and exposure to light. - Prepare Permeabilization Solution:
- 999 mL PBS
- 1 mL Triton™ X-100 (0.1% v/v)
- 5 mL TWEEN® 20 (0.5% v/v)
Note: Permeabilization Solution without serum may be stored at room temperature for 1 year. - Prepare Citrate Buffer:
- 1 L dH2O
- 2.94 g sodium citrate dihydrate
- Mix thoroughly then adjust the pH to 6.0 by adding HCl
- 500 µL TWEEN® 20
Note: Citrate Buffer may be stored at 2 - 8°C for 6 months.
Fixation
- Remove medium from cell culture inserts
- Wash both sides of the inserts with PBS (200 µL apical, 500 µL basolateral)
- Add 4% PFA to the cell culture inserts: 200 µL apical side, 500 µL basolateral side
- Incubate 30 minutes at room temperature
- Remove PFA from cell culture inserts
- Wash 2x with PBS as in step 2
- Cultures can be stored in PBS at 4°C for up to one month (seal plate with parafilm to prevent evaporation), but it is better to proceed to the next step as soon as possible to minimize loss of antigen signal
Antigen Retrieval
- Remove PBS
- Add citrate buffer to both sides of the cell culture inserts
- Heat entire plate to 98°C for 20 minutes (can be done in vegetable steamer or other type of heating block device—as long as water does not enter the plate or inserts)
- Allow plate to cool at room temperature
- Remove citrate buffer and wash 2x with PBS
Staining
- All staining steps are performed inside of the cell culture inserts.
- Block the cells by adding 200 µL (apical) and 500 µL (basolateral) of blocking solution to the inserts. Blocking solution is prepared by adding 10% donkey serum to the Permeabilization Solution. Seal plate with parafilm and incubate overnight at 4°C.
- Prepare primary antibody solution by adding antibodies to the Permeabilization Solution. Antibodies are the same as for organoid cultures as listed in the Technical Manual:
- Remove blocking solution and add primary antibody solution (200 µL apical 400 µL basolateral) to the inserts. Incubate overnight or for up to 2 days at 4°C.
Table 1. Recommended Primary Antibodies for Immunocytochemistry
ANTIBODY CATALOG # ISOTYPE DILUTION Primary antibodies Anti-RAGE/AGER R&D Systems AF1145 Goat IgG 1 in 400 Anti-GPRC5a Sigma HPA007928 Rabbit IgG 1 in 500 Anti-human HT2-280 Terrace Biotech TB-27AHT2-280 Mouse IgM 1 in 200 Anti-Pro-SPC Sigma AB3786 Rabbit IgG 1 in 2000 - Remove primary antibody solution and wash 3x with Permeabilization Solution
- Prepare secondary antibody solution using Permeabilization Solution with 10% donkey serum.
Note: The secondary antibody must be raised against the same isotype as the primary. For example, if using mouse IgM as your primary you will need an anti-mouse IgM for your secondary.
- Incubate inserts in secondary antibody solution (200 µL apical 500 µL basolateral) in the dark on a rocker at room temperature for 2 hours
- Remove secondary antibody solution and wash 3x with Permeabilization Solution
- Counterstain with DAPI (2 µg/mL) for 20 min at room temperature in the dark
- Remove DAPI stain and wash 3x with PBS
- Carefully cut the membranes from the hangers of the inserts using a scalpel and remove with forceps
- Mount, using preferred mounting medium, on slides with the apical side facing the coverslip
- Allow mountant to cure as recommended by the manufacturer before imaging
Flow Cytometry
- Remove medium from cell culture inserts
- Wash cell culture inserts with PBS (200 µL apical 500 µL basolateral)
- Add 200 µL ACF Enzymatic Dissociation Solution to each well
- Incubate for 10 minutes at 37°C
- Monitor inserts for rounding of cells or lifting from inserts
- Gently scrape cells from inserts with pipette tip
- Pipette up and down to remove all cells from inserts
- Add cells to microcentrifuge tube
- Add 200 µL ACF Enzyme Inhibition Solution to the microcentrifuge tubes
- Centrifuge at 400 x g for 5 minutes to pellet cells
- Resuspend cells in medium or PBS and continue flow cytometry procedure as linked in the Technical Manual.

Figure 3. Alveolar Cells Grown on Cell Culture Inserts Maintain Expression of HT2-280 and Increase Expression of HT1-56.
ATII cells from three separate donors were seeded from organoid domes onto ECM-coated cell culture inserts and cultured to Day 7. Expression of the ATII marker HT2-280 and the ATI marker HT1-56 was determined as the percentage of viable singlets by flow cytometry. The data demonstrates HT2-280 high positive expression decreases slightly but not significantly, while HT1-56 high positive expression increases significantly (ANOVA with Dunnett’s post hoc test, where ** = p < 0.01; n = 3) when alveolar cells are cultured on cell culture inserts compared to organoid cultures. There were no differences between ALI (3 days) and always submerged cultures in either HT2-280 or HT1-56 expression. Symbols represent different donors across the conditions.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration