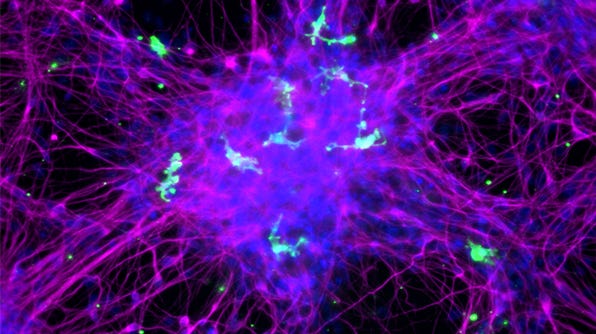
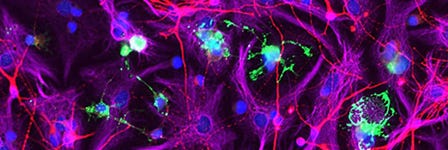
How to Co-Culture Human Pluripotent Stem Cell (hPSC)-Derived Forebrain Neurons and Microglia
The central nervous system (CNS) and its resident immune cells, the microglia, develop from different embryonic germ layers. As a result, microglia do not intrinsically arise in most in vitro neural differentiation protocols, requiring the use of co-cultures to model neuroimmune interactions. This protocol describes a method for co-culturing human pluripotent stem cell (hPSC)-derived microglia and forebrain neurons by generating each cell type separately and then combining them under optimized culture conditions. This protocol aims for a final density of 5% microglia, which is the reported microglia density in the human cortex1,2,3.
Important Notes:
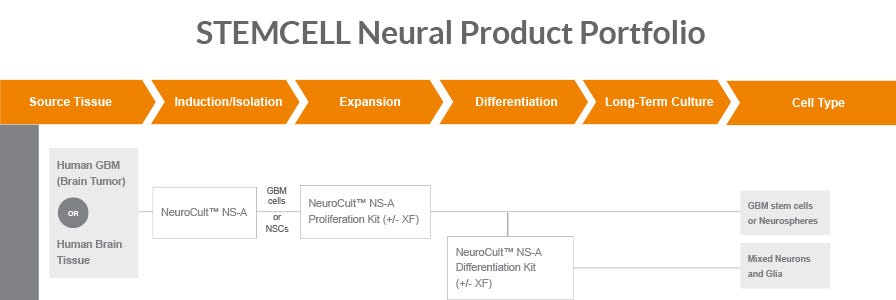
- This protocol involves differentiation of hPSCs to microglia using STEMdiff™ Hematopoietic Kit followed by STEMdiff™ Microglia Differentiation Kit. In parallel, hPSCs are differentiated to forebrain neuron precursors using STEMdiff™ Forebrain Neuron Differentiation Kit and matured for at least 7 days with STEMdiff™ Forebrain Neuron Maturation Kit. These forebrain neurons are then combined with microglia in co-culture.
- There are multiple workflow options to generate these two cell types in parallel prior to combining them in co-culture. For details, please refer to the relevant Product Information Sheet (PIS) for STEMdiff™ Microglia Differentiation and Maturation Kits and for STEMdiff™ Forebrain Neuron Differentiation and Maturation Kits.
- The recommended co-culture period is 3 - 10 days. To ensure optimal microglia survival, do not exceed 10 days of culture following the 24-day microglia differentiation (e.g. steps 1 - 8 in section A under 'Directions for Use' in the STEMdiff™ Microglia PIS).
- Cells may be cryopreserved at various points for increased flexibility in co-culture planning (please contact techsupport@stemcell.com for instructions).
Materials
- STEMdiff™ Microglia Differentiation Kit (Catalog #100-0019)
- STEMdiff™ Hematopoietic Kit (Catalog #05310)*
- STEMdiff™ Forebrain Neuron Differentiation Kit (Catalog #08600)
- STEMdiff™ Forebrain Neuron Maturation Kit (Catalog #08605)
- STEMdiff™ SMADi Neural Induction Kit (Catalog #08581)✝
- The following required materials are offered together as the BrainPhys™ hPSC Neuron Kit (Catalog #05795):
- BrainPhys™ Neuronal Medium, 500 mL (Catalog #05790)
- NeuroCult™ SM1 Neuronal Supplement, 10 mL (Catalog #05711)
- N2 Supplement-A, 5 mL (Catalog #07152)
- Human Recombinant BDNF, 10 µg (Catalog #78005)
- Human Recombinant GDNF, 10 µg (Catalog #78058)
*Required for microglia differentiation as outlined in the PIS’s for STEMdiff™ Hematopoietic Kit and for STEMdiff™ Microglia kits.
✝Required for forebrain-type neuron differentiation as outlined in the PIS for STEMdiff™ Forebrain Neuron kits.
Protocol
This procedure has been optimized for use with hPSC maintenance reagents and multiple embryonic stem (ES) and induced pluripotent stem (iPS) cell lines. For upstream protocols and source materials, please see the mTeSR™ Plus Technical Manual and the Product Information Sheet for STEMCELL’s highly quality-controlled Healthy Control Human iPSC Line, Female, SCTi003-A.
Part I. Differentiate hPSCs to Microglia
- Generate hematopoietic progenitor cells (HPCs) by following the entire protocol outlined in the PIS for STEMdiff™ Hematopoietic Kit.
- Evaluate efficiency of HPC differentiation by flow cytometry. The resulting cell population should be > 90% CD43+ and > 20% positive for coexpression of CD34/CD45.
- Under "Directions for Use" in the PIS for STEMdiff™ Microglia Differentiation & Maturation Kits, follow section A steps 1 - 8 (Microglia Differentiation).
- Evaluate efficiency of microglia differentiation by flow cytometry. The resulting cell population should be > 80% positive for coexpression of CD45 and CD11b.
- Optional: Microglia may be further cultured using STEMdiff™ Microglia Maturation Kit (Catalog #100-0020) prior to co-culture setup in Part III. However, do not exceed 10 days in total of additional culture time after microglia differentiation (total of maturation and co-culture period combined).
Part II: Differentiate hPSCs to Forebrain Neurons
- Follow either the embryoid body (EB) or monolayer protocol outlined in the PIS for STEMdiff™ Forebrain Neuron kits.
Note: When seeding neuronal precursors into STEMdiff™ Forebrain Neuron Maturation Medium (section C, step 1 under "Directions for Use" in the PIS), the suggested density for co-culture with microglia ranges from 1.5 x 104 - 4 x 104 cells/cm2. The optimal density should be determined by the user.
- Continue the forebrain neuron maturation phase until neurons have been cultured in STEMdiff™ Forebrain Neuron Maturation Medium for at least 7 days.
- Optional: If desired, the forebrain neuron maturation period may be extended prior to co-culture setup in Part III, by following the instructions in the PIS for STEMdiff™ Forebrain Neuron kits.
- Verify successful forebrain neuron differentiation by performing immunocytochemistry on a subset of cultured cells. The resulting cell population should be > 90% positive for ꞵIII-tubulin and FOXG1, and < 10% positive for the astrocyte marker GFAP.
Part III: Prepare Optimized Microglia-Neuron Co-Culture Medium and Set Up Co-Culture
- In the PIS for BrainPhys™, follow the instructions under ‘Preparation of Complete Differentiation Medium’ (section B) to prepare the required volume of BrainPhys™ Neuronal Medium + supplements.
Note: The required volume of medium will depend on the plate format and desired length of the co-culture period.
- Thaw STEMdiff™ Microglia Supplement 2 (Component #100-0023 of STEMdiff™ Microglia Differentiation Kit) at room temperature (15 - 25°C) until just thawed, or alternatively at 2 - 8°C overnight. Mix thoroughly.
Note: If not used immediately, aliquot supplement and store at -20°C. Do not exceed the expiry date (EXP) as indicated on the label.
Note: If you do not have enough leftover STEMdiff™ Microglia Supplement 2 to prepare the optimized co-culture medium, please contact techsupport@stemcell.com to purchase this component individually.
- Prepare optimized Microglia-Neuron Co-Culture Medium as follows:
a. Add STEMdiff™ Microglia Supplement 2 (250X) to the BrainPhys™ Neuronal Medium + supplements to a final concentration of 1X (e.g. 40 µL of supplement per 10 mL of medium).
b. Mix thoroughly.
Note: If not used immediately, store Microglia-Neuron Co-Culture Medium at 2 - 8°C for up to 2 weeks.
- Collect microglia from Part I:
a. Transfer the entire cell suspension to a 15 mL conical tube. To ensure all cells are collected, additional washes may be performed using warm DMEM/F-12 with 15 mM HEPES.
b. Centrifuge at 300 x g for 5 minutes.
c. Remove supernatant and resuspend the pellet in an appropriate volume of Microglia-Neuron Co-Culture Medium.
d. Count cells using Trypan Blue and a hemocytometer.
- Dilute the microglia cell suspension in additional Microglia-Neuron Co-Culture Medium to obtain the required final cell concentration and volume.
- The concentration and volume of the final microglia suspension should be optimized by the user and will depend on the desired microglia-to-forebrain neuron ratio, the number of culture wells used, the plate format, and the initial cell density of forebrain neuronal precursors plated in Part II.
- The recommended seeding density for microglia in co-culture is half of the forebrain neuron precursor density seeded in Part II, Step 1 (e.g. a 1:2 microglia-to-neuronal precursor ratio). This will result in microglia comprising approximately 5% of the final co-culture cell population after 7 days.
Note: The decrease in proportion of microglia in the final co-culture is attributable to forebrain neuronal precursors proliferating to a small degree during early maturation, failure of all seeded microglia to adhere, unavoidable loss of semi-adherent microglia during subsequent medium changes, and a small proportion of microglia undergoing cell death.
- If relatively high forebrain neuronal precursor proliferation is observed during early forebrain neuron maturation, the microglia seeding density may be increased to achieve the desired proportion of microglia in the final co-culture after 7 days.
- The relative percentage of microglia-to-neurons differs between brain regions1,2,3, and the seeding densities of both cell types may be adjusted accordingly as desired.
- Remove and discard the culture medium from the forebrain neurons generated in Part II.
- Seed the microglia suspension prepared in Part III, Step 6 onto the forebrain neurons.
- Place the plate in a 37°C and 5% CO2 incubator. Distribute cells evenly by moving the plate in several quick, short, back-and-forth and side-to-side motions.
- Maintain the co-cultures for 3 - 10 days by performing a half-medium change every 2 - 3 days using Microglia-Neuron Co-Culture Medium prepared in Part III, step 3.
Note: Microglia are semi-adherent and may unintentionally be removed during medium changes. Prior to performing half-medium changes, centrifuge the plate in a swinging-bucket centrifuge with plate adaptors at 100 x g for 2 minutes to force the cells to settle.
Characterize by Immunocytochemistry
- Immunocytochemistry may be performed after 3-10 days of co-culture, using the following primary antibodies:
- Neuronal marker: Mouse anti-tubulin β 3 (Clone TUJ1, BioLegend Catalog #801202)
- Microglia marker: Rabbit polyclonal anti-IBA1 (Synaptic Systems Catalog #234 003)
- Astrocyte marker: Chicken polyclonal anti-GFAP (Aves Labs Catalog #GFAP)
- Total cell density may be assessed by DAPI staining.
- Expected observations: Cultures should consist of healthy microglia integrated among forebrain neurons, with < 10% astrocytes present. Microglia should display an unactivated, ramified morphology with extended cellular processes.
References
- Mittelbronn M et al. (2001) Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol 101(3): 249–55.
- Von Bartheld CS et al. (2016) The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J Comp Neurol 524(18): 3865–95.
- Agarwal D et al. (2020) A single-cell atlas of the human substantia nigra reveals cell-specific pathways associated with neurological disorders. Nat Commun 11(4183): 1–11.
- Document #PR00053
- Version 1.0.0
- June 2021
- Immunocytochemistry may be performed after 3-10 days of co-culture, using the following primary antibodies:
Microglia Seeding Density Considerations
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration