STEMdiff™ Hematopoietic - EB Supplement B
Animal component-free culture supplement for hematopoietic differentiation optimized for lymphoid potential
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
-
 STEMdiff™ Hematopoietic - EB Basal Medium
STEMdiff™ Hematopoietic - EB Basal MediumSerum-free medium for the generation of embryoid bodies for downstream lymphoid differentiation
-
 STEMdiff™ Hematopoietic - EB Supplement A
STEMdiff™ Hematopoietic - EB Supplement AAnimal component-free culture supplement for mesoderm specification with downstream lymphoid potential
-
 AggreWell™400
AggreWell™400Microwell culture plates for easy and reproducible production of embryoid bodies and spheroids
-
Labeling Antibodies
Compatible antibodies for purity assessment of isolated cells
Overview
Available either individually or as part of the STEMdiff™ NK Cell Kit or STEMdiff™ T Cell Kit, where it can be used to promote the differentiation of hPSCs to NK or T cells, respectively.
For detailed information on the two differentiation protocols, please explore the STEMdiff™ NK Cell Kit Technical Manual or the STEMdiff™ T Cell Kit Technical Manual.
Data Figures
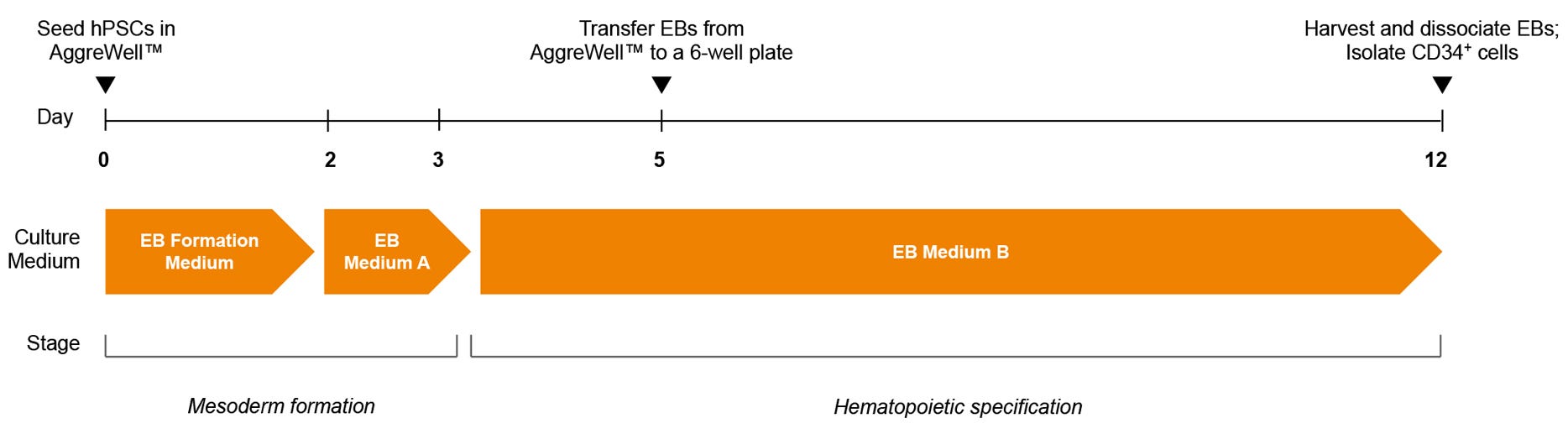
Figure 1. STEMdiff™ Hematopoietic-EB Progenitor Differentiation Protocol
hPSCs are harvested and dissociated into a single-cell suspension prior to seeding into AggreWell™ plates in EB Formation Medium (EB Medium A + 10 µM Y-27632) to form 600-cell aggregates. A half-medium change with EB Medium A is performed on day 2. After a total of 3 days of mesoderm formation, the medium is changed to EB Medium B to induce hematopoietic lineage differentiation. On day 5, EBs are transferred onto non-tissue culture-treated plates. After a total of 12 days, EBs are harvested and dissociated, then CD34+ cells are enriched by EasySep™ positive selection.
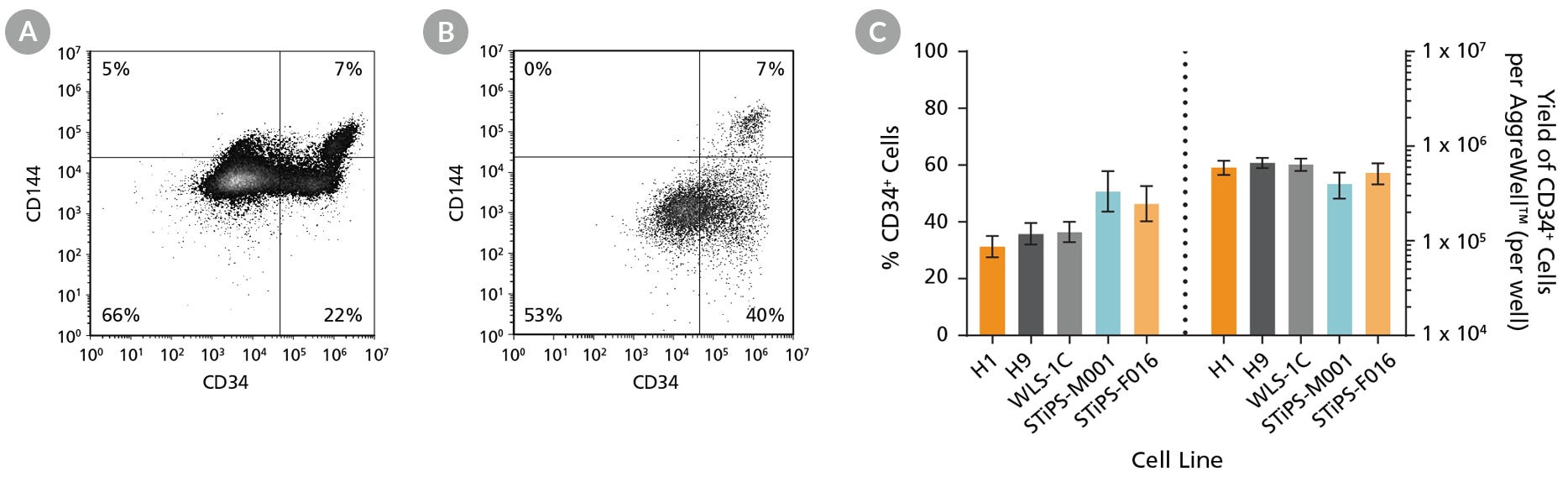
Figure 2. hPSCs Differentiate to CD34+ Hematopoietic Progenitor Cells After 12 Days of Culture
Human ES and iPS cells were induced to differentiate to CD34+ cells using the 12-day protocol shown in Figure 1. At the end of the culture period, cells were harvested, dissociated into a single-cell suspension, and analyzed by flow cytometry for CD34 and CD144 expression. Dead cells were excluded by light-scatter profile and DRAQ7™ staining. Representative flow cytometry plots for (A) ES (H1) cell-derived and (B) iPS (STiPS-M001) cell-derived cells analyzed on day 12 are shown. (C) The average frequency of viable CD34+ cells on day 12 (before CD34+ cell isolation) for two ES cell lines (H1 and H9) and three iPS cell lines (WLS-1C, STiPS-M001, and STiPS-F016) ranged between 31% and 51%. The average yield of CD34+ cells produced per well of a 6-well AggreWell™400 plate ranged between 4.0 x 105 and 6.7 x 105. Data are shown as mean ± SEM (n = 9 - 35).
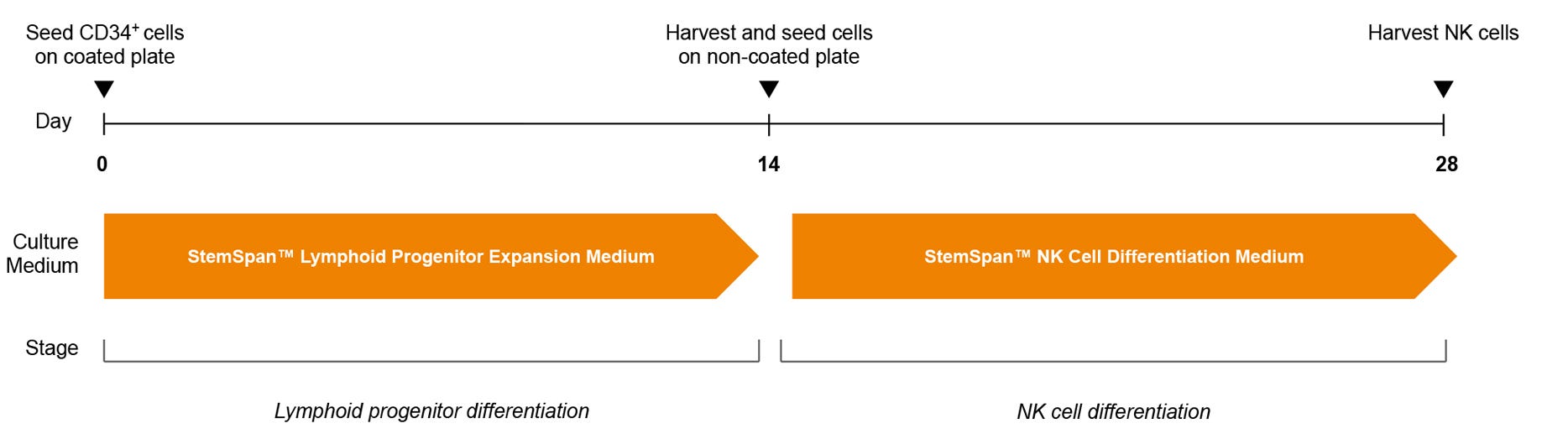
Figure 3. NK Cell Generation Protocol
hPSC-derived CD34+ cells are seeded in StemSpan™ Lymphoid Progenitor Expansion Medium on plates coated with StemSpan™ Lymphoid Differentiation Coating Material. On day 14, cells at the lymphoid progenitor stage are harvested and reseeded in StemSpan™ NK Cell Differentiation Medium onto non-coated plates for further differentiation to NK cells.
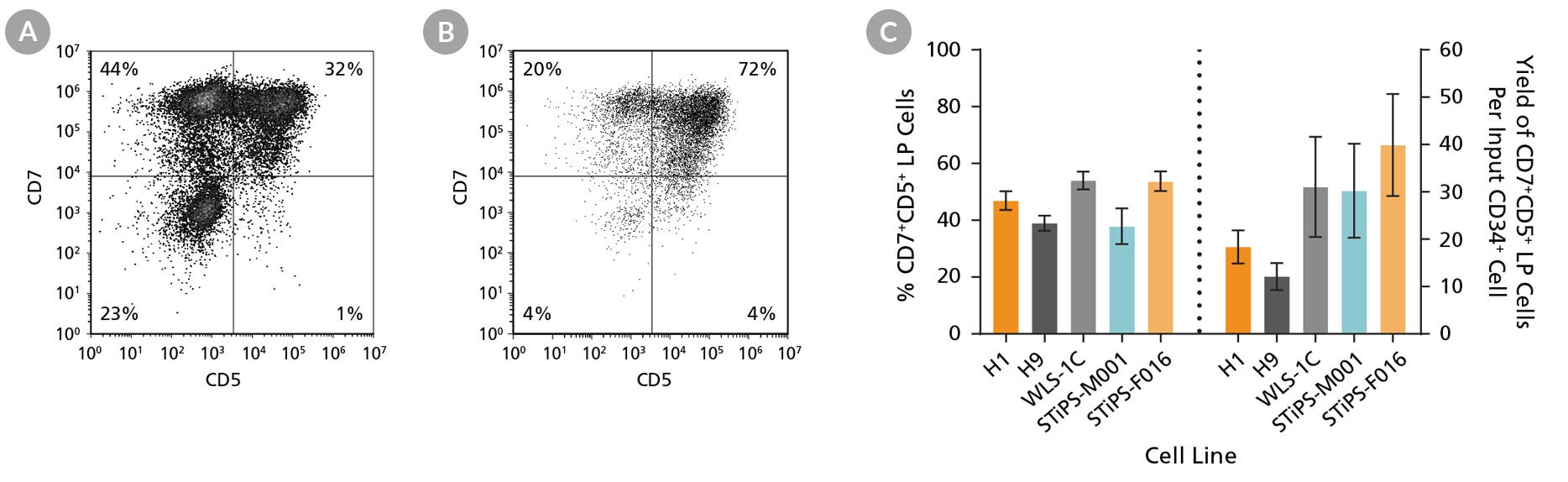
Figure 4. hPSC-Derived CD34+ Cells Differentiate to CD5+CD7+ Lymphoid Progenitor Cells Over 14 Days of Culture
hPSC-derived CD34+ cells were cultured for 14 days in StemSpan™ Lymphoid Progenitor Expansion Medium on plates coated with StemSpan™ Lymphoid Differentiation Coating Material (Figure 2). Cells were harvested and analyzed for CD7 and CD5 expression by flow cytometry. Representative flow cytometry plots for (A) ES (H1) cell-derived and (B) iPS (STiPS-M001) cell-derived cells are shown. (C) The average frequency of viable CD7+CD5+ lymphoid progenitor cells on day 14 ranged between 38% and 54%, and the average yield of lymphoid progenitor cells produced per input hPSC-derived CD34+ cell was between 12 and 40. Data are shown as mean ± SEM (n = 8 - 32).
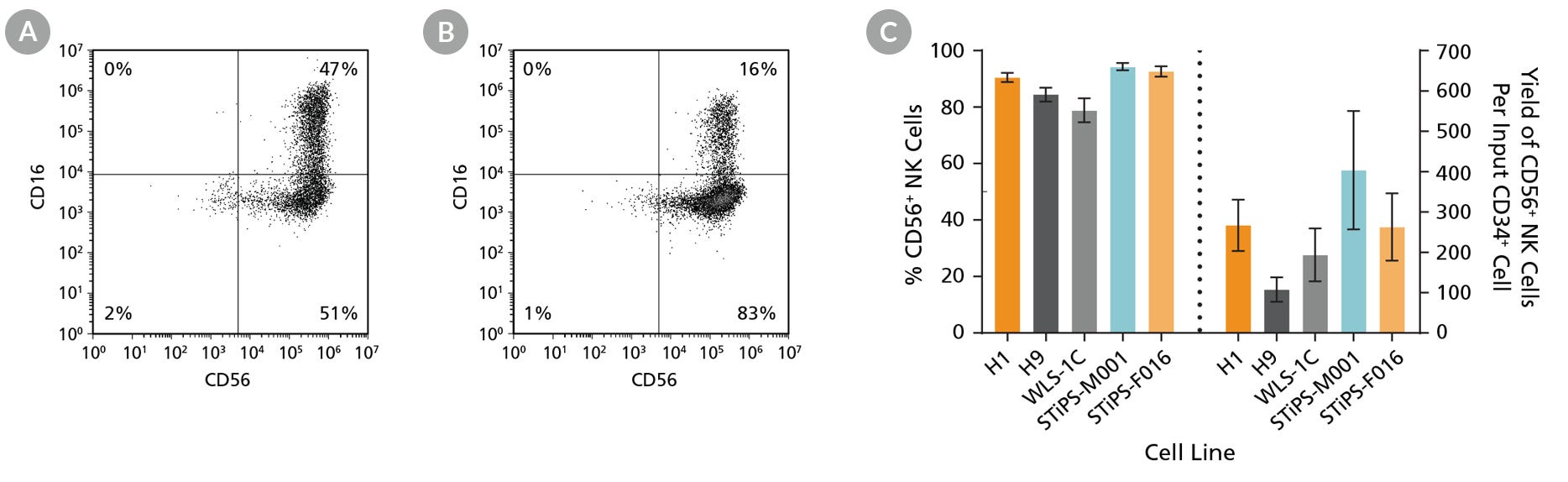
Figure 5. hPSC-Derived Lymphoid Progenitor Cells Differentiate to CD56+ NK Cells After 14 Days of Culture
hPSC-derived lymphoid progenitor cells were cultured in StemSpan™ NK Cell Differentiation Medium on non-coated plates for 14 days (Figure 2). Cells were harvested and analyzed for expression of CD56 and CD16 by flow cytometry. Representative flow cytometry plots are shown for both (A) ES (H1) cell-derived and (B) iPS (STiPS-M001) cell-derived cells. (C) After 28 days of culture, the average frequency of viable CD56+ NK cells from hPSC-derived CD34+ cells ranged between 79% and 94%. The average yield of CD56+ cells produced per hPSC-derived CD34+ cell was between 108 and 404. Data are shown as mean ± SEM (n = 7 - 18).
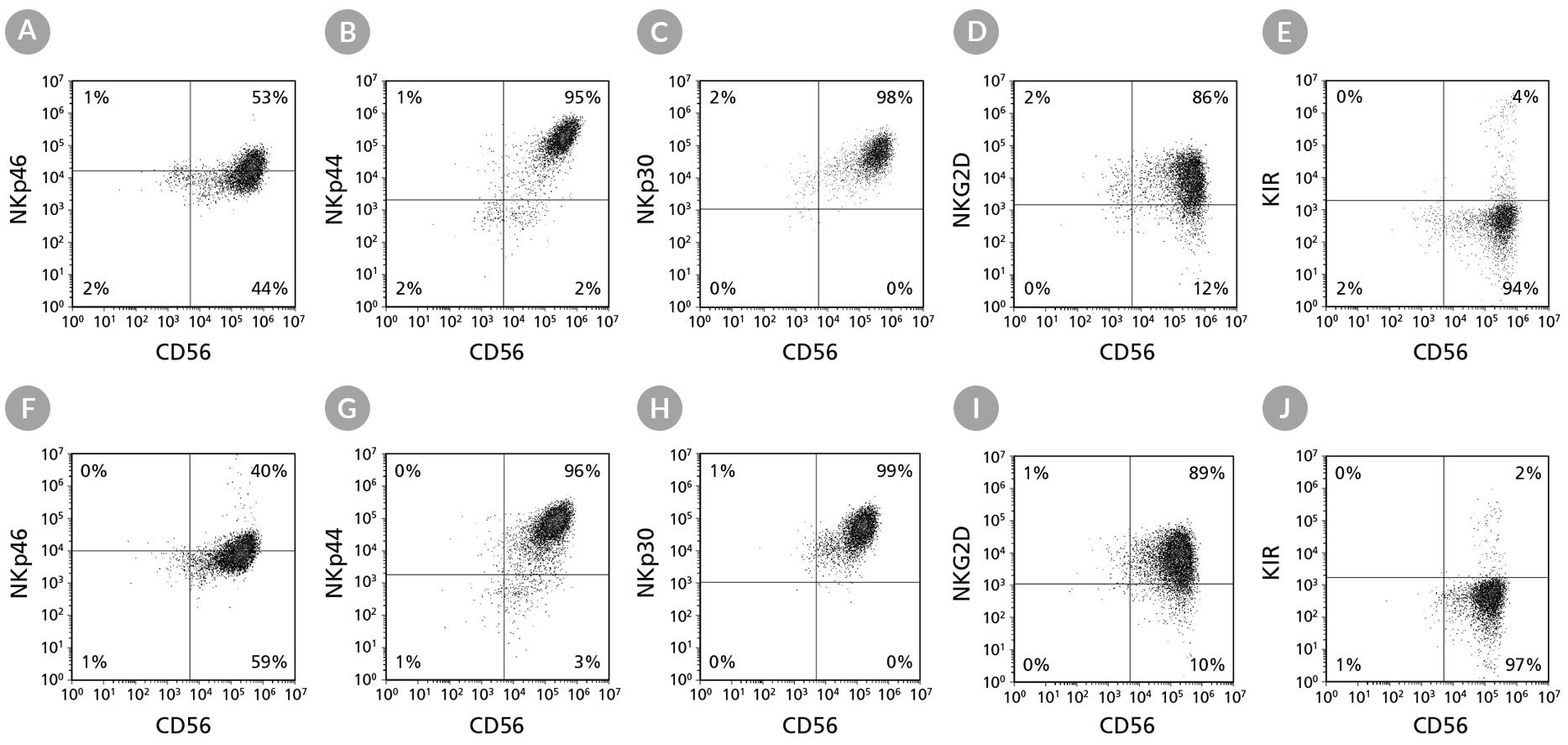
Figure 6. Cell Surface Marker Expression on PSC-Derived CD56+ NK Cells After 28 Days of Culture
hPSC-derived CD34+ cells were cultured in StemSpan™ Lymphoid Progenitor Expansion Medium on plates coated with StemSpan™ Lymphoid Differentiation Coating Material for 14 days, followed by 14 days of culture in StemSpan™ NK Cell Differentiation Medium on non-coated plates to generate CD56+ NK cells (Figure 2). Cells were harvested and analyzed for CD56, NKp46, NKp44, NKp30, NKG2D, and KIR expression by flow cytometry. Dead cells were excluded by light-scatter profile and DRAQ7™ staining. Data shown are from representative cultures initiated with (A-E) ES (H1) cells or (F-J) iPS (STiPS-M001) cells.
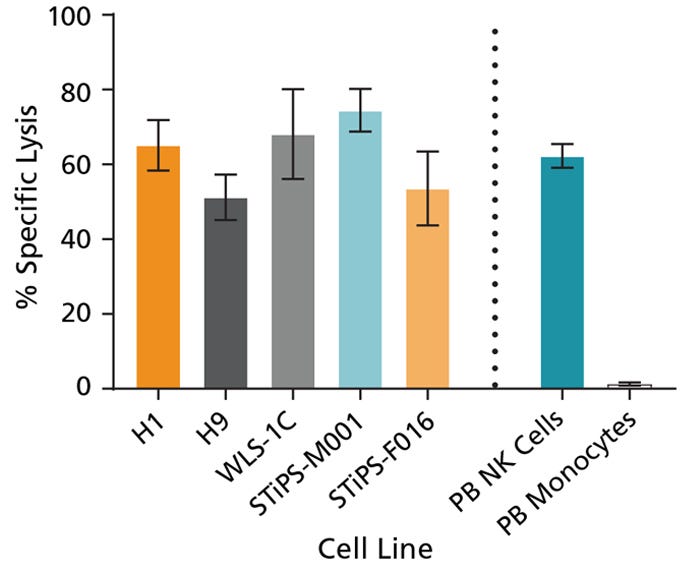
Figure 7. Cultured NK Cells Exhibit Cytotoxicity Toward K562 Cell Line
hPSC-derived CD56+ NK cells were co-cultured with calcein AM (CAM)-labeled K562 cells at a ratio of 2.5:1 for 4 hours. Isolated peripheral blood (PB) NK cells and monocytes were also co-cultured with labeled K562 cells as positive and negative controls, respectively. Before the co-culture, frozen PB NK cells were thawed and cultured overnight with StemSpan™ NK Cell Differentiation Supplement and StemSpan™ SFEM II, while PB monocytes were cultured overnight in SFEM II only. To measure maximum release, the labeled K562 cells were treated with 1% Triton™ X-100. Culture supernatants were assessed for fluorescence released by dead cells after 4 hours using a SpectraMax® microplate reader (excitation 485 nm/emission 520 nm). The % specific lysis was calculated as follows: [(test release - spontaneous release) / (maximum release - spontaneous release)] x 100%. The average specific lysis by hPSC- derived NK cells ranged between 51% and 75% as compared to 62% specific lysis by PB NK cells. Cultures of ES (H1 and H9) and iPS (WLS-1C, STiPS-M001, and STiPS-F016) cells are shown. Data are shown as mean ± SEM (n = 3 - 7).
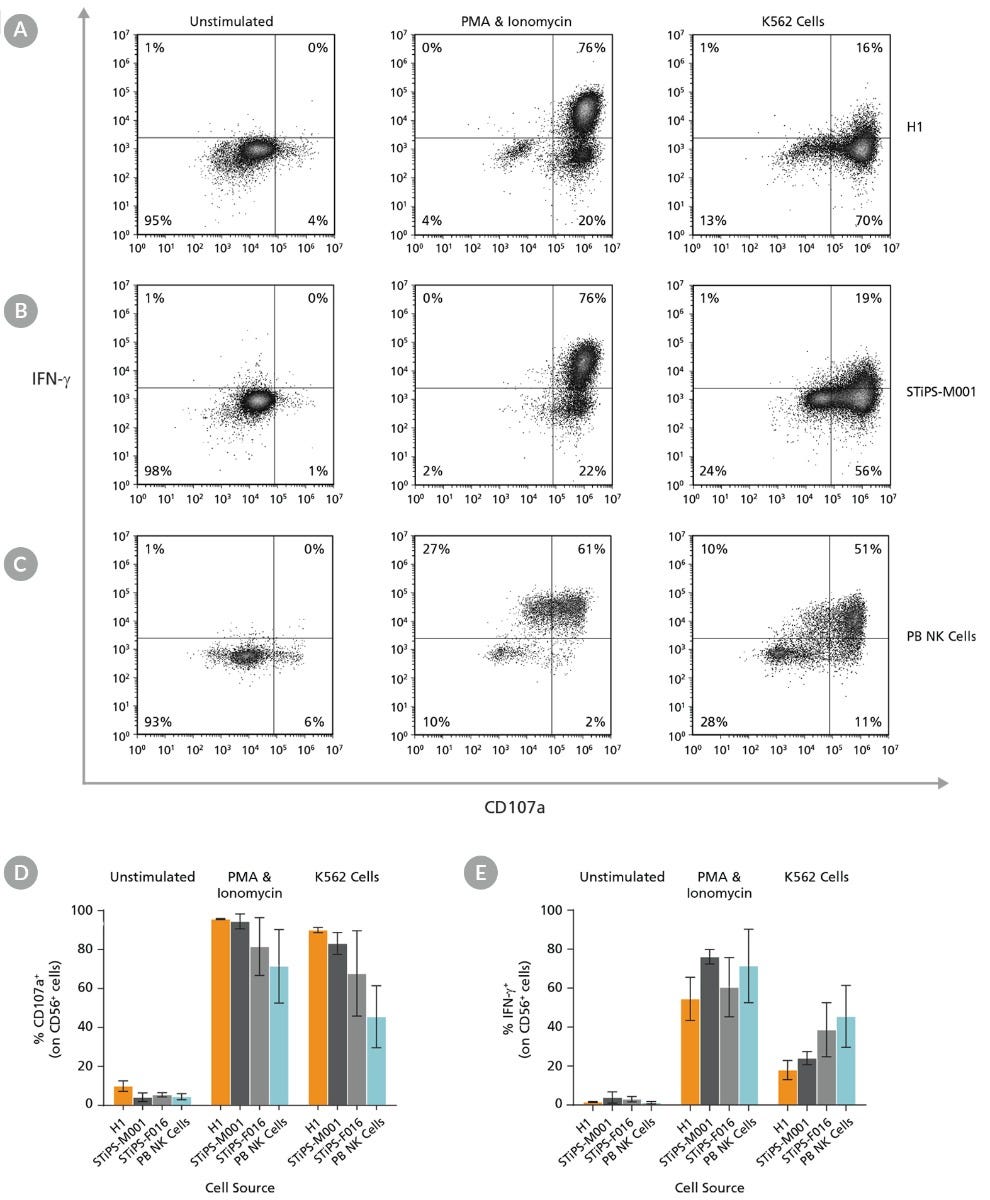
Figure 8. Cultured hPSC-Derived CD56+ NK Cells Are Induced to Express Surface CD107a and Produce IFN-γ After Co-Culture with K562 Target Cells or Stimulation with PMA and Ionomycin
hPSC-derived NK cells were obtained using STEMdiff™ NK Cell Kit as described. hPSC-derived CD56+ NK cells were left unstimulated or were stimulated with either phorbol 12-myristate 13-acetate (PMA) and ionomycin or with K562 cells at a ratio of 1:1 effector to target cells. CD107a antibody and stimulation factors were added to each well. After one hour, monensin and brefeldin A were added to each well to inhibit protein transport. After a total incubation time of 4 hours at 37°C, cells were washed and stained with Zombie NIR™ Fixable Viability Kit and CD56. Cells were then fixed, permeabilized, and stained for IFN-γ. Surface CD107a and intracellular IFN-γ expression were assessed using flow cytometry. Representative samples are gated on CD56. (A) Representative unstimulated, PMA & ionomycin- stimulated, and K562-stimulated ES (H1) cell-derived NK cell samples. (B) Representative unstimulated, PMA & ionomycin-stimulated, and K562-stimulated iPS (STiPS-M001) cell-derived NK cell samples. (C) Representative unstimulated, PMA & ionomycin-stimulated, and K562-stimulated PB NK cell samples. The PB NK cell sample was thawed and cultured overnight in StemSpan™ SFEM II supplemented with IL-2 prior to this assay. (D) Summary of CD107a and (E) IFN-γ expression results by NK cells. Upon stimulation, hPSC-derived CD56+ NK cells are able to degranulate, as shown by surface expression of CD107a (average range: 82 - 96% for PMA & ionomycin stimulation and 68 - 90% for K562 stimulation) and secrete IFN-γ (54 - 76% and 18 - 39% for PMA & ionomycin and K562 stimulation, respectively). Data are shown as mean ± SEM (n = 2 - 4 ).
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Resources and Publications
Educational Materials (11)
Related Products
Item added to your cart

STEMdiff™ Hematopoietic - EB Supplement B
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.