AggreWell™ Microwell Plates - Spheroids and Embryoid Bodies
AggreWell™ Microwell Plates
Easy, Reproducible 3D Spheroid and Embryoid Body Production
AggreWell™ plates contain microwells that are 400 µm (AggreWell™400), 800 µm (AggreWell™800) or 900 µm (AggreWell™HT) in size, providing flexibility to generate spheroids of desired size for your research.
How Does AggreWell™ Work?
Watch this short video to learn more about how AggreWell™ works to generate uniformly-sized and shaped 3D spheroids and embryoid bodies.
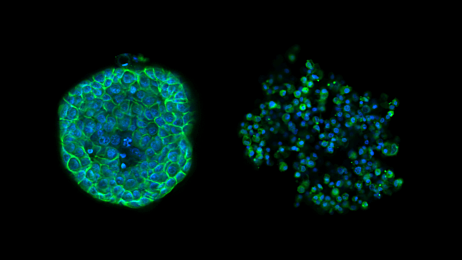
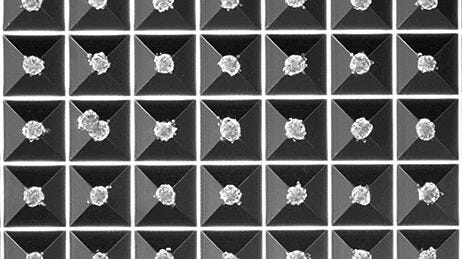
To produce spheroids or embryoid bodies using AggreWell, simply add a single cell suspension to the microwells, and then centrifuging to distribute the cells evenly in the microwells. Incubate overnight and assess the spheroids after 24-48 hours. Then culture in the microwells, or harvest and perform downstream assays.
Why Use AggreWell™ to Generate EBs and Spheroids?
- Use a simple protocol to generate spheroids and EBs.
- Produce large numbers of uniform spheroids, with consistent size and shape.
- Control the size of the spheroids by modifying the cell seeding concentration.
- Obtain up to 5,900 spheroids per well.
- Scale up production of spheroids and EBs from a single plate for a fraction of a penny per spheroid.
Scientific Resources
Key Applications and Related Publications
ES and iPS Cell Directed Differentiation (Selected)
Cheung et al. (2014) Telomerase protects werner syndrome lineage-specific stem cells from premature aging. Stem Cell Reports 2(4):534-46
Hartjes et al. (2014) Selection via pluripotency-related transcriptional screen minimizes the influence of somatic origin on iPSC differentiation propensity. Stem Cells 32(9):2350-9
Jang et al. (2014) Culture of Pig Induced Pluripotent Stem Cells without Direct Feeder Contact in Serum Free Media. J Stem Cell Res Ther 4(2):174
Kinney et al. (2014) Mesenchymal morphogenesis of embryonic stem cells dynamically modulates the biophysical microtissue niche. Sci Rep 4: 4290
Kokkinaki M. (2011) Human Induced Pluripotent Stem-Derived Retinal Pigment Epithelium (RPE) Cells Exhibit Ion Transport, Membrane Potential, Polarized Vascular Endothelial Growth Factor Secretion, and Gene Expression Pattern Similar to Native RPE. STEM CELLS 29:825–835
Nguyen et al. (2014) Microscale Generation of Cardiospheres Promotes Robust Enrichment of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cell Reports 3(2): 260–268
Sebastiano V et al. (2014) Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med 6(264):264ra163
Ungrin et al. (2012) Rational bioprocess design for human pluripotent stem cell expansion and endoderm differentiation based on cellular dynamics. Biotechnol Bioeng 109(4): 853-66
van Wilgenburg et al. (2013) Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PloS one 8(8):e71098
Cancer Spheroid Research
Razian et al. (2013) Production of Large Numbers of Size-controlled Tumor Spheroids Using Microwell Plates. J Vis Exp. 81: 50665
Wrzesinski et al. (2014) The Cultural Divide: Exponential Growth in Classical 2D and Metabolic Equilibrium in 3D Environments. PLoS ONE 9(9): e106973
Drug Screening & Drug Delivery
Bratt-Leal A et al. (2013). A microparticle approach to morphogen delivery within pluripotent stem cell aggregates. Biomaterials 34(30): 7227-35
Fey et al. (2012) Determination of drug toxicity using 3D spheroids constructed from an immortal human hepatocyte cell line. Toxicol Sci 127(2): 403-11
Lim et al. (2011) Development of nano- and microscale chondroitin sulfate particles for controlled growth factor delivery. Acta Biomater 7(3): 986-95
Lei et al. (2014) Characterization of a multilayer heparin coating for biomolecule presentation to human mesenchymal stem cell spheroids. Biomater Sci 2: 666-673
Disease Modeling
Aflaki et al. (2014) Macrophage models of Gaucher disease for evaluating disease pathogenesis and candidate drugs. Sci Transl Med. 11;6(240): 240ra73
Moya et al. (2013) An integrated model of perfused tumor and cardiac tissue. Stem Cell Research & Therapy, 4(Suppl 1):S15
3D Tissue Engineering
Cho et al. (2013) Generation of human secondary cardiospheres as a potent cell processing strategy for cell-based cardiac repair. Biomaterials 34(3): 651-61
Cimetta et al. (2014) Microscale technologies for regulating human stem cell differentiation. Exp Biol Med. 239(9):1255-63
Kabiri et al. (2012) 3D mesenchymal stem/stromal cell osteogenesis and autocrine signalling. Biochem Biophys Res Commun 419(2): 142-7
Suspension Culture of MSCs
Baraniak et al. (2012) Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 347(3): 701-11
Cook et al. (2012) Micromarrows - Three-dimensional coculture of hematopoietic stem cells and mesenchymal stromal cells. Tissue Engineering Part C: Methods 18(5): 319-328
Rettinger et al. (2014) In vitro characterization of scaffold-free three-dimensional mesenchymal stem cell aggregates. Cell Tissue Res 358(2): 395-405
Additional Applications
Wallace et al. (2013) Using 3D culture to investigate the role of mechanical signaling in keratinocyte stem cells. Methods Mol Biol 989: 153-64
AggreWell™ Background
Ungrin et al. (2008) Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One 3(2) e1565