Maestro Edge™
Multiwell multi-electrode array (MEA) system with 384 channels
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
-
 CytoView MEA™ Plate
CytoView MEA™ PlateMultiwell multi-electrode array (MEA) plate, black or white polystyrene walls with transparent SU-8 bottom; 6-, 24-, 48-, 96-well formats
-
 BioCircuit MEA™ Plate
BioCircuit MEA™ PlateMultiwell multi-electrode array (MEA) plate, clear polystyrene walls with an opaque bottom; 24-, 48-, 96-well formats
-
 Software Modules for Maestro MEA™ Systems
Software Modules for Maestro MEA™ SystemsData acquisition and analysis modules for Maestro Pro™ and Edge™ MEA systems
-
Labeling Antibodies
Compatible antibodies for purity assessment of isolated cells
Overview
The Maestro Edge™ is equipped with 384 channels that enable simultaneous live recordings from up to 24 wells at once. The smart environmental chamber ensures an optimal environment for cells and for recording by providing precise temperature and CO2 control while minimizing electrical and mechanical noise. Data acquisition and analysis are simplified with intuitive neural and cardiac software modules, allowing you to quickly translate complex functional activity data into clear results and publication-ready figures.
The Maestro Edge™ is compatible with CytoView MEA™ Plates and BioCircuit MEA™ Plates in 6- and 24-well formats. Confidently achieve robust and physiologically meaningful data by using the Maestro Edge™ MEA system in combination with our specialty cell culture media (e.g. BrainPhys™, neural STEMdiff™ kits, cardiac STEMdiff™ kits), high-quality iPSCs and iPSC-derived cells, and accessory reagents that support functionally active cultures. For information about our instrument services, including warranty and service packages, contact us at www.stemcell.com/info-maestro-pro.
If you are looking to conduct higher-throughput experiments, the Maestro Pro™ is compatible with 6-, 24-, 48-, and 96-well plates.
Data Figures

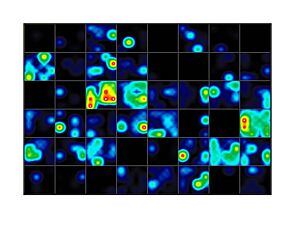
Figure 1. hPSC-Derived Neurons Demonstrate Measurable Network Activity Recorded on the Maestro MEA™ System
(A) hPSC-derived neurons cultured in BrainPhys™ Neuronal Medium (Catalog #05790) were plated on the Maestro MEA™ System. (B) The neurons became electrically active over a 15-week period, with a gradual increase in MFR from 0.18 ± 0.05 Hz at Week 8 to 3.68 ± 0.47 Hz at Week 16 (n = 1; mean ± SEM, 128 electrodes). (C) Raster plots show the firing patterns of the neurons across 64 electrodes at different time points. Each black line represents a detected spike. Each blue line represents a single channel burst, a collection of at least 5 spikes, each separated by an ISI of ≤ 100 ms. Each pink box indicates a network burst, a collection of at least 10 spikes from a minimum of 25% participating electrodes across the entire well, each separated by an ISI of ≤ 100 ms. Neurons cultured in BrainPhys™ Neuronal Medium demonstrate electrical activity as shown by the increased number of spikes over time. In addition, an increase in network bursting frequency was observed, suggesting that neuronal firing gradually organized into synchronized network bursts as the neurons matured. MEA = microelectrode array; MFR = mean firing rate; ISI = inter-spike interval

Figure 2. Human iPSC-Derived Forebrain Neuron Precursor Cells Increase Neuronal Activity Over 42 Days in Culture
Human iPSC-Derived Forebrain Neuron Precursor Cells (Catalog #200-0770) were generated from the hiPSC line SCTi003-A (Catalog #200-0511). The neuron precursors were then matured on a 48-well CytoView MEA™ plate (Catalog #200-0870) with STEMdiff™ Forebrain Neuron Maturation Kit (Catalog #08605). Electrical activity from 16 electrodes was measured over time using the Maestro MEA™ System. (A - D) Detected spikes (black lines), single channel bursts (blue lines; a collection of at least 5 spikes, each separated by an ISI of no more than 100 ms), and network bursts (orange boxes; a collection of at least 50 spikes from a minimum of 35% of participating electrodes, each separated by an ISI of no more than 100 ms) were recorded for each timepoint. Neuronal activity can be detected by Day 14 and increases over time throughout the 42-day culture period. (E) Mean firing rate, (F) number of bursts, and (G) synchrony index were all shown to increase over the 42-day culture period. hiPSC = human induced pluripotent stem cell; MEA = microelectrode array; ISI = inter-spike interval

Figure 3. The Maestro MEA™ System Enables Functional Evaluation of hPSC-Derived Cardiomyocytes and Assessment of Electrophysiological Responses to Pharmacological Stimuli
(A) Ventricular cardiomyocytes were derived from four hPSC lines (H1, H9, 1C, F016) using the STEMdiff™ Ventricular Cardiomyocyte Differentiation Kit (Catalog #05010) and maintained using the STEMdiff™ Cardiomyocyte Maintenance Kit (Catalog #05020). At day 25, ventricular cardiomyocytes demonstrate a characteristic MEA electrophysiology profile, including large spike amplitude, small repolarization waveform, and stable beating frequency. (B) MEA recordings of hPSC-derived cardiomyocytes (Day 27) show characteristic electrical profiles and drug response to drug response to E4031 and Nifedipine (10 nM and 300 nM, respectively; gray lines). E4031 prolonged and Nifedipine shortened the repolarization, respectively. For guidance on how to dissociate and plate hPSC-derived cardiomyocytes for MEA Assays, view our online protocol. MEA = multi-electrode array
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Resources and Publications
Educational Materials (15)
Related Products
-
 STEMdiff™ Ventricular Cardiomyocyte Differe...
STEMdiff™ Ventricular Cardiomyocyte Differe...Serum-free media for differentiation of human PSCs to ventricular cardiomyocytes and long-term maintenance of human PSC-derived cardiomyocytes
-
 BrainPhys™ Neuronal Medium
BrainPhys™ Neuronal MediumSerum-free neurophysiological basal medium for improved neuronal function
-
 STEMdiff™ Forebrain Neuron Differentiation ...
STEMdiff™ Forebrain Neuron Differentiation ...Differentiation kit for the generation of neuronal precursors from human ES and iPS cell-derived neural progenitor cells
-
 STEMdiff™ Atrial Cardiomyocyte Differentiat...
STEMdiff™ Atrial Cardiomyocyte Differentiat...Serum-free culture medium kit for differentiation of human PSCs to atrial cardiomyocytes
-
 Healthy Control Human iPSC Line, Female, SCTi...
Healthy Control Human iPSC Line, Female, SCTi...Human pluripotent stem cell line, frozen
-
 Human iPSC-Derived Forebrain Neuron Precursor...
Human iPSC-Derived Forebrain Neuron Precursor...Frozen human forebrain neuron precursor cells differentiated from human induced pluripotent stem cell line, SCTi003-A
-
 Maestro Pro™
Maestro Pro™High-throughput multiwell multi-electrode array (MEA) system with 768 channels
Item added to your cart

Maestro Edge™
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.
















