Gene Editing Human Peripheral Blood Natural Killer Cells using the CellPore™ Transfection System
Natural Killer (NK) cells are innate immune cells capable of killing tumor cells or virus-infected cells without prior antigen exposure. Genome editing of NK cells has been a key area of focus in the field of cell therapy to enhance their functionality or introduce target specificity (e.g. CAR-NK). Despite significant advancements, editing NK cells remains challenging due to their sensitivity to manipulation and limited proliferative capacity. NK cell transfection typically requires a lengthy process that necessitates expansion periods both before and after transfection. While pre-expansion can increase gene editing efficiency, it can also alter NK cell characteristics and impact their function. Eliminating pre-expansion can be beneficial for faster turnaround times and maintaining NK cell characteristics, but it requires careful optimization for high efficiency.
This simple, streamlined protocol presents an innovative approach to address some of these challenges using the mechanoporation-based, gentle delivery technology of the CellPore™ Transfection System. It includes step-by-step instructions for the isolation of human primary NK cells from peripheral blood sources, preparation and delivery of Cas9 ribonucleoprotein (RNP) complexes, and recommendations for post-transfection handling, culture, and analysis. When used under optimized conditions, the CellPore™ Transfection System can enable robust knock-out efficiency in resting NK cells while preserving cell phenotype and function.
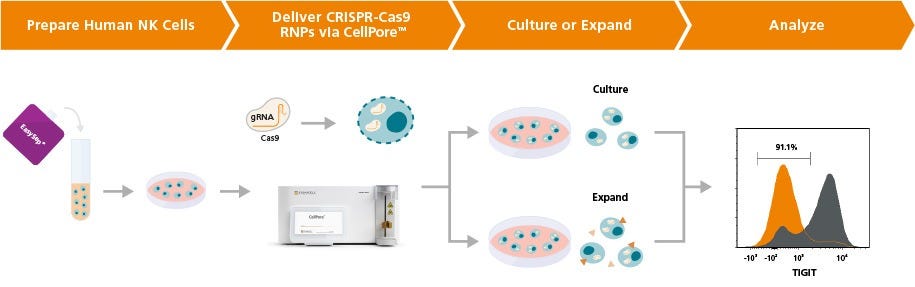
Figure 1. Experimental Workflow for Gene Editing of Human NK Cells Using the CellPore™ Transfection System
Human primary NK cells can be isolated from peripheral blood sources using EasySep™ (Catalog #17955). Cells are initially cultured overnight in ImmunoCult™ NK Cell Base Medium supplemented with Human Recombinant IL-2. CRISPR-Cas9 RNPs are prepared by complexing glycerol-free Cas9 nuclease with ArciTect™ sgRNAs at an optimal ratio. RNPs are delivered to NK cells via the CellPore™ Transfection System. Post-transfection, cells are returned to culture and subsequently analyzed for editing efficiency via established methods, as permitted by the experimental design, such as flow cytometry or ArciTect™ T7 Endonuclease I Kit (Catalog #76022). Edited NK cells can also be expanded downstream over 14 days using the ImmunoCult™ NK Cell Expansion Kit (Catalog #100-0711).
Materials
- CellPore™ Transfection System (Catalog #100-0946)
- CellPore™ Transfection Kit 300 (Catalog #100-1020)
- Cas9 Nuclease (glycerol-free recommended)
- Target gRNA (e.g. ArciTect™ sgRNA; Catalog #200-0013)
- ImmunoCult™ NK Cell Base Medium (Catalog #100-0712)
- Human Recombinant IL-2 (Catalog #78145)
- EasySep™ Human NK Isolation Kit (Catalog #17955)
- EasySep™ Buffer (Catalog #20144)
- Nuclease-free water (Catalog #79001)
Part I: Sample Preparation
For available fresh human whole peripheral blood and peripheral blood leukopaks, refer to Catalog #70504 or #70500, respectively.
PERIPHERAL BLOOD
Prepare a peripheral blood mononuclear cell (PBMC) suspension from whole blood by centrifugation over a density gradient medium (e.g. Lymphoprep™; Catalog #18060). For faster PBMC preparation, use SepMate™ RUO (Catalog #86450 or #86415) or SepMate™ IVD* (Catalog #85450 or #85415) cell isolation tubes.
After preparation, resuspend cells at 5 x 107 cells/mL in EasySep™ Buffer. Proceed to part II.
LEUKAPHERESIS
Wash the peripheral blood leukapheresis sample by adding an equivalent volume of EasySep™ Buffer or phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS). Centrifuge at 300 x g for 10 minutes at room temperature (15 - 25°C). Remove the supernatant and resuspend the cells at 5 x 107 cells/mL in EasySep™ Buffer. Proceed to part II.
Part II: NK Cell Isolation and Pre-Transfection Culture
- Pre-warm a sufficient volume of ImmunoCult™ NK Cell Base Medium to 37°C.
Note: Optional: ImmunoCult™ NK Cell Base Medium can be supplemented with 10 ng/mL human recombinant IL-2.
- Isolate human NK cells from the sample (prepared in Part I) using the EasySep™ Human NK Cell Isolation Kit following the instructions in the Product Information Sheet (Document #10000005299).
- Centrifuge isolated cells at 500 x g for 5 minutes at room temperature (15 - 25°C).
- Resuspend isolated cells in pre-warmed (37°C) ImmunoCult™ NK Cell Base Medium at 1 - 2 x 106 cells/mL.
- Culture isolated cells overnight in a conical tube or culture plate in a humidified incubator at 37°C and 5% CO2.
Part III: Preparation of sgRNA Working Solution
- Briefly centrifuge the vial of lyophilized sgRNA before opening.
- Add nuclease-free water to the vial to achieve a final concentration of 100 μM (see Table 1 for examples). Mix thoroughly.
Note: If not used immediately, aliquot the sgRNA working solution into DNase- and RNase-free microtubes and store at -20°C for up to 6 months. Alternatively, store at -80°C for long-term storage. After thawing the aliquots, use immediately. Do not refreeze.
Table 1. Resuspension of sgRNA to 100 μM
sgRNA (nmol) Volume of Nuclease-Free Water (μL) 1.5 15 10 100 50 500 *100 μM is equal to 100 pmol/μL
Part IV: Preparation of CRISPR-Cas9 RNP Complex
The following example is for preparing RNP complexes for 1 reaction. Adjust accordingly based on the number of reactions required.
- To prepare the RNP Complex Mixture, combine the components listed in Table 2 in a sterile DNase- and RNase-free microcentrifuge tube. Adjust volumes according to the number of reactions required, including controls.
Note: A glycerol-free formulation of Cas9 is required for optimal performance.ote: A glycerol-free formulation of Cas9 is required for optimal performance.Table 2. Preparation of RNP Complex Mixture
Reagent For 0.5 - 10 x 106 cells For > 10 - 20 x 106 cells Volume (μL) Amount (pmol) Volume (μL) Amount (pmol) 10 mg/mL Cas9 Nuclease (Glycerol-Free Formulation) 0.64 40 1.28 80 100 μM sgRNA 1.00 100 2.00 200 CellPore™ Delivery Medium 8.36 – 6.72 – Total 10.00 – 10.00 – Note: The above example provides the required volumes for a 1:2.5 Cas9:sgRNA ratio. It is recommended to optimize the Cas9:sgRNA ratio and Cas9 amount for each gene target (see Tips for Further Optimization). - Mix thoroughly by gently pipetting up and down. Avoid introducing excessive bubbles.
- Incubate RNP Complex Mixture at room temperature (15 - 25°C) for 15 minutes.
Note: If not used immediately after incubation, keep on ice until use. Allow the RNP Complex Mixture to warm to room temperature for 5 minutes prior to transfection.
Part V: Preparation of Reaction Mixture for Transfection
Each CellPore™ Delivery Cartridge 300 can process 5 x 105 to 2 x 107 primary NK cells per reaction. The following example is for preparing one Reaction Mixture (primary NK cells + RNP Complex Mixture + [optional] CellPore™ FITC-Dextran). Adjust volumes accordingly based on the number of reactions required. Include a small excess to account for pipetting error.
- Prepare a sufficient volume of complete culture medium depending on the required number of reactions and cell culture wells by supplementing ImmunoCult™ NK Cell Base Medium with 10 ng/mL recombinant human IL-2 and 5% human AB serum. Warm to 37°C.
Note: If desired, add antibiotics immediately before use (e.g. 50 μg/mL gentamicin).
- Harvest cells from the culture plate (Part II, Step 5) by gently pipetting up and down to resuspend cells that have settled down. Transfer cells to a 15 mL conical tube.
- Perform a viable cell count to determine concentration. Transfer the desired number of primary NK cells for each reaction to a sterile DNase- and RNase-free microcentrifuge tube.
- Centrifuge at 500 x g for 5 minutes at room temperature (15 - 25°C).
- Aspirate the supernatant completely and resuspend primary NK cells in CellPore™ Delivery Medium as follows:
- For 5 x 105 - 1 x 107 cells, resuspend in 40 µL.
- For > 1 x 107 - 2 x 107 cells, resuspend in 90 µL.
Note: Optionally, CellPore™ FITC-Dextran may be co-delivered as a positive control to measure successful cargo delivery. Adjust the resuspension volume to accommodate for the additional volume of CellPore™ FITC-Dextran if applicable (Table 3). - Add 10 µL of RNP Complex Mixture (prepared in Part IV) to the resuspended cells (with or without CellPore™ FITC-Dextran) according to Table 3.
- Gently mix the Reaction Mixture by pipetting up and down. Immediately proceed to Part VI.
Table 3. Volumes for Preparing 50 or 100 μL of Reaction Mixture
Component For 5 x 105 - 1 x 107 cells For > 1 x 107 - 2 x 107 cells Volume (μL) Volume with CellPore™ FITC-Dextran (μL) Volume (μL) Volume with CellPore™ FITC-Dextran (μL) Resuspended Primary NK Cells 40 37.5 90 85 RNP Complex Mixture 10 10 10 10 CellPore™ FITC-Dextran – 2.5 - 5 Total volume 50 100
Part VI: Delivery of RNP Complexes to Primary NK Cells Using the CellPore™ Transfection System
- Remove the Cartridge Insert of a new CellPore™ Delivery Cartridge 300 (Figure 2) and add complete culture medium (prepared in Part V, Step 1) to the Collection Tube. Re-insert the Cartridge Insert into the Collection Tube.
- For 5 x 105 - 1 x 107 cells, add 150 μL
- For > 1 x 107 - 2 x 107 cells, add 100 μL
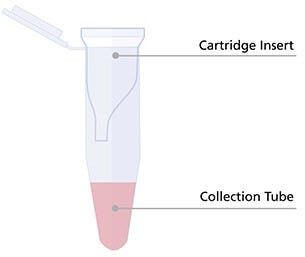
Figure 2. Preload the Collection Tube of the CellPore™ Delivery Cartridge with Complete Culture Medium.
- Transfer the entire volume of the Reaction Mixture (50 µL or 100 µL) into the Cartridge Insert. Always insert the pipette tip to the bottom of the Cartridge Insert when dispensing the sample (Figure 3).
Note: Do not centrifuge the Delivery Cartridge at this stage as this will lead to a reduction in delivery performance. Gently tap the Delivery Cartridge several times to collect the volume at the bottom if necessary.
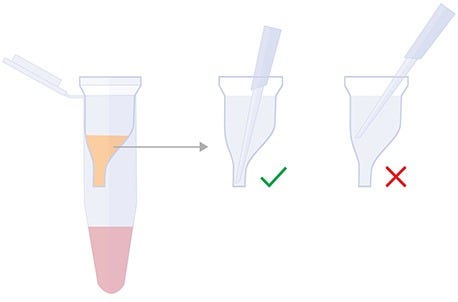
Figure 3. Proper Pipetting Technique for CellPore™ Delivery Cartridge
- Close the cap of the Cartridge Insert and ensure it is securely placed in the Collection Tube.
- Place the Delivery Cartridge into the Cartridge Holder of the CellPore™ Transfection System instrument.
- Set the instrument pressure to 70 psi and the run time to 3 seconds. Press Run.
Note: For complete instructions on performing sample runs, refer to the CellPore™ Transfection System User Reference Manual (Document #10000018433)
- Once the run is complete, retrieve the Delivery Cartridge from the instrument. The cell sample should be at the bottom or side of the collection tube.
Note: It is recommended to spin down the Delivery Cartridge in a mini-centrifuge for a few seconds for full volume recovery.
- Remove and discard the Cartridge Insert. Perform a viable cell count.
- Transfer the cell suspension to an appropriate culture plate and add pre-warmed complete culture medium (prepared in Part V, Step 1) to the desired culturing density. Incubate cells in a humidified incubator at 37°C with 5% CO2 until ready for analysis or downstream applications.
Note: If proceeding with NK cell expansion, cells can immediately be transferred to coated plates. Refer to the ImmunoCult™ NK Cell Expansion Kit Product Information Sheet for guidance.
Part VII: Assessing Viability and Delivery Efficiency
The following fluorochrome-conjugated antibodies and dyes are recommended to facilitate analysis of gene-edited NK cells:
- Anti-Human CD45 Antibody, Clone HI30 (Catalog #60018)
- Anti-Human CD3 Antibody, Clone UCHT1 (Catalog #60011)
- Anti-Human CD56 Antibody, Clone HCD56 (Catalog #60021)
- Anti-Human CD16 Antibody, Clone 3G8 (Catalog #60041)
- Viability Dye, including 7-AAD (Catalog #75001), or Propidium Iodide (Catalog #75002)

Figure 4. The CellPore™ Transfection System Enables Efficient Gene Knockout in Human NK Cells
Fresh primary NK cells were cultured in ImmunoCult™ NK Base Medium supplemented with 10 ng/mL IL-2 overnight. RNP complexes were delivered to 2 x 106 NK cells per reaction following the optimized delivery parameters. After a culture period of at least 5 days, cells were analyzed by flow cytometry to determine gene target knockout efficiency. (A) Representative histograms of surface marker expression and knockout for Cas9 RNP targeting TIGIT, FCGR3, KLRC1, or NCR1 genes. (B) Average surface marker knockout efficiency reached above 80% for TIGIT (via the TIGIT gene), NKG2A (via the KLRC1 gene), and NKp46 (via NCR1 gene), while CD16 knockout (via the FCGR3 gene) reached above 60%. (C) All conditions maintained high viability relative to the untreated sample, and (D) comparable levels of CD56dim/CD16+ and CD56bright/CD16- NK cell subsets relative to untreated samples. Data are presented as mean ± SD, n = 3 - 8.
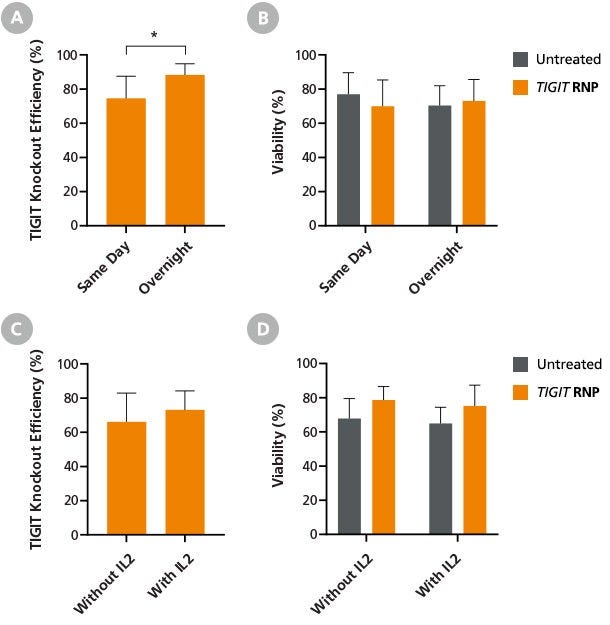
Figure 5. Overnight Culture Before Transfection Improves Primary NK Cell Editing Efficiency
Fresh primary NK cells were cultured for 1 - 2 hours (Same Day) or overnight in ImmunoCult™ NK Base Medium supplemented with 10 ng/mL IL-2. RNP complexes targeting the TIGIT gene were delivered to 2 x 106 NK cells per reaction following the optimized delivery parameters. Cells were analyzed by flow cytometry after a minimum of 4 days post-transfection to allow sufficient time for surface marker knockout. (A) TIGIT knockout efficiency was significantly higher (88.3 ± 6.5%) in cells cultured overnight (i.e. 16 - 20 hours) compared to same-day cultured cells (74.6 ± 12.9%; p = 0.019, paired t-test). (B) Viability was similar for all culture regimens. (C) The addition of 10 ng/mL Human Recombinant IL-2 in the ImmunoCultTM NK Base Medium during overnight culture provided marginal improvements to TIGIT knockout efficiency. (D) NK cell viability was comparable between samples cultured with and without IL-2. Data are presented as mean ± SD, n = 2-5.
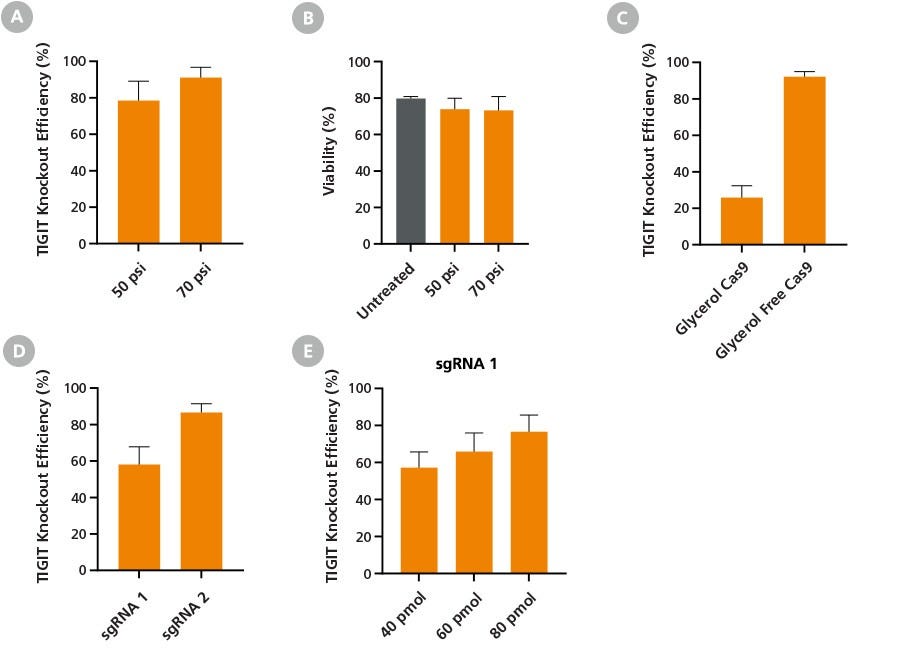
Figure 6. Optimization of CRISPR-Cas9 RNP Delivery, Composition, and Dosage, for Efficient Gene Knockout Using the CellPore™ Transfection System
Fresh primary NK cells were cultured overnight in ImmunoCult™ NK Base Medium supplemented with 10 ng/mL Human Recombinant IL-2. Cas9 RNP complexes targeting the TIGIT gene were delivered to 2 x 106 NK cells per reaction following the optimized delivery parameters unless otherwise stated. Cells were analyzed by flow cytometry after a minimum of 4 days post-transfection to allow sufficient time for surface marker knockout. (A) Maximum TIGIT knockout efficiency was achieved at 70 psi, with (B) comparable viability to untreated cells. (C) NK cells were transfected with RNP complexes prepared using either a glycerol-free or glycerol-containing Cas9 formulation at the optimized 70 psi delivery condition. Glycerol-free Cas9 formulation enabled optimal editing efficiency, increasing TIGIT knockout efficiency from 26% to 92%. Furthermore, (D) TIGIT sgRNA design was optimized by evaluating knockout efficiency between two different sequences. When used at the same dose (40 pmol), sgRNA 2 outperformed sgRNA 1. (E) Rescue of sgRNA 1 knockout performance was achieved by increasing the amount of RNP added to the reaction. Data are presented as mean ± SD, n = 2 - 4.
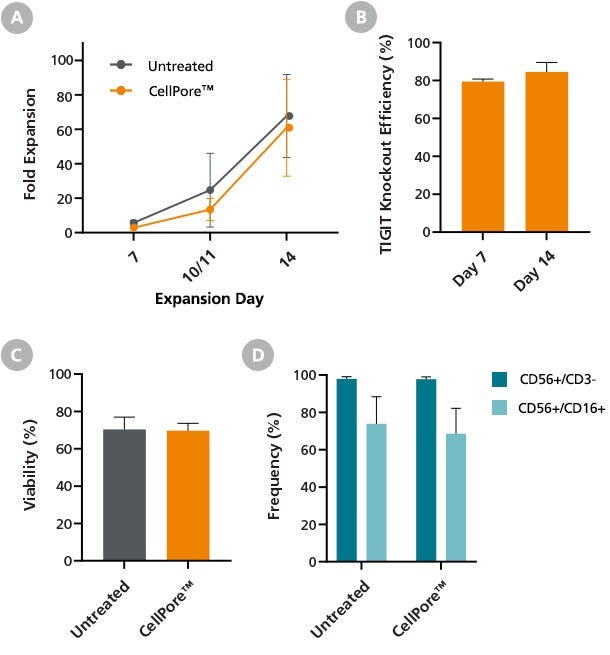
Figure 7. Primary NK Cells Manipulated by the CellPore™ Transfection System Retain Their Expansion Potential
Gene-edited human primary NK cells were expanded for 14 days using the ImmunoCult™ NK Expansion Kit immediately post-transfection with TIGIT RNPs. NK cells were cultured in ImmunoCult™ NK Cell Expansion Medium on plates coated with ImmunoCult™ NK Cell Expansion Coating Material. On Day 7, and again on Day 10 or 11, expanding NK cells were harvested and re-seeded on freshly coated plates. (A) Total fold expansion at Day 14 was comparable between gene-edited NK cells manipulated by CellPore™ compared to untreated cells. Importantly, (B) flow cytometry assessment of TIGIT surface marker expression confirmed sustained knockout efficiency throughout the 14-day expansion period. Additionally, the (C) viability and (D) phenotype of 14-day expanded gene-edited cells was comparable to untreated NK cells. Data are presented as mean ± SD, n = 4.
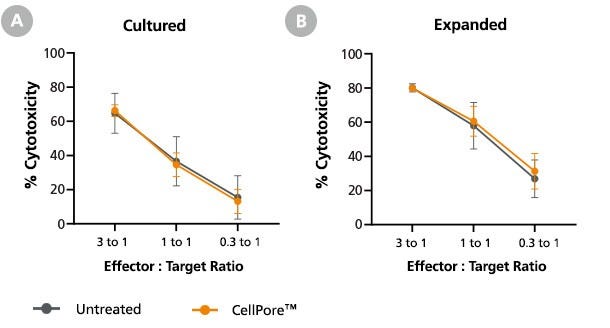
Figure 8. Primary NK Cells Manipulated by the CellPore™ Transfection System Retain Their Cytotoxicity
NK cells were mechanoporated using the CellPore™ Transfection System without cargo delivery in order to assess the impact of mechanoporation on NK cell cytotoxic function. The cells were either (A) cultured for 5 days in complete medium or (B) expanded for 14 days using the ImmunoCultTM NK Expansion Kit. Following the culture period, NK cells were harvested and co-cultured with K562 cells at different effector-to-target ratios at 37°C for 4 hours. K562 target cell lysis was measured via flow cytometry, staining with Annexin V (for detection of apoptotic cells) and 7-aminoactinomycin D (7-AAD; for detection of necrotic cells). Cytotoxic function of mechanoporated NK cells was comparable to an untreated control for either culture regimen. Data are presented as mean ± SD, n = 2 - 3.
Tips for Further Optimization
- This protocol is not recommended for transfecting expanded human NK cells.
- This protocol is not optimized for working from cryopreserved NK cells. It is recommended to isolate NK cells from fresh PBMCs.
- In case cell clumping is observed prior to transfection, it is recommended to filter aggregated suspensions through a 37 µm cell strainer (e.g. Catalog #27250) for optimal results.
- The culture period before NK cell transfection can be reduced from overnight to a minimum of 1 hour for a shorter transfection protocol. This may result in lower editing efficiencies.
- Longer pre-transfection cultures can enhance editing efficiency for some target genes. This may result in lower cell viability and recovery. NK cells should not be cultured for more than 3 days prior to use with the CellPore™ Transfection System.
- Best results are obtained when limiting prolonged cell exposure to ambient temperature conditions. Consider keeping unused cells in a humidified incubator with 5% CO2 at 37°C when performing larger experiments.
- Titration of Cas9 RNPs may be required to obtain optimal editing efficiencies. Similarly, titration of the Cas9:sgRNA ratio (from 1:1 - 1:8) may also be required.
- For best results, the total volume of cargo added should not exceed 10% of the reaction volume.
- Reducing the reaction volume to less than 50 µL may result in lower editing efficiencies and cell recoveries. A minimum reaction volume of 50 µL is required for consistent performance with the CellPore™ Transfection System.
- An optional rest period after transfection of up to 2 hours at 37°C can be implemented before transferring cells to an appropriate culture system.
- For applications where downstream cytotoxic function is critical, it is recommended to keep NK cells in culture for a minimum of 4 days before harvesting. Earlier time points may result in impacts to downstream function.
- Lowering the instrument pressure to 50 or 60 psi can improve the functionality of cells in certain applications. However, this may result in lower gene editing efficiencies.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration

