Co-Culturing Colorectal Organoids and T Cells using IntestiCult™ and ImmunoCult™
- Document # 27247
- Version 1.2.0
- Apr 2024
Background
Colorectal cancer is the third most commonly diagnosed cancer (10.0% of all total cases) and the second highest leading cause of cancer death (9.4%) worldwide.1 Scientists currently rely on immortalized cell lines and animal models to translate knowledge from basic research into clinical therapeutics; however, neither of these models accurately recapitulate the true tumor niche.2 Gastrointestinal organoid models have demonstrated their capacity to better mimic the corresponding tissue environment and can be used to model tumor initiation and progression, and test therapeutic response.3
Organoid-T cell co-cultures are emerging as practical, in vitro models for evaluating novel therapeutics for immuno-oncology. Patient-derived organoids (PDOs) provide physiologically relevant preclinical data, enabling patient-specific evaluation of response to targeted treatments. While optimal culture conditions for both PDOs and T cell cultures have been developed, challenges remain to identify culture conditions that preserve the viability and functionality of both cell types with the same media.
In this technical bulletin, we describe the co-culture of colorectal (CRC)-derived PDOs and T cells using IntestiCult™ Organoid Growth Medium (Human) and ImmunoCult™-XF T Cell Expansion Medium. For complete instructions, use this document in coordination with the IntestiCult™ and ImmunoCult™ Product Information Sheets (PIS; Document #DX21423 and #DX20347). The IntestiCult™ PIS provides detailed information, including a list of materials and instructions for isolating human colonic crypts from biology samples, establishing human intestinal organoids from the isolated crypts, and expanding and maintaining organoid cultures via passaging. The ImmunoCult™-XF PIS provides detailed information, including a list of materials and instructions for expanding activated human T cells.
Materials
- IntestiCult™ Organoid Growth Medium (Human) (Catalog #06010) or IntestiCult™ OGM Human Basal Medium (Catalog #100-0190)
- ImmunoCult™-XF T Cell Expansion Medium (Catalog #10981)
- Human IFN-γ ELISA Kit (Catalog #02003)
- Gentle Cell Dissociation Reagent (Catalog #100-1077)
- CellTiter-Glo® 3D Cell Viability Assay (Promega Benelux BV. Catalog #G9683)
- GloCell™ Fixability Viability Dye Red 780 (Catalog #75007)
- Corning® Matrigel® Matrix, Growth Factor Reduced (GFR), Phenol Red-Free (e.g. Corning #356231)
- DMEM/F-12 with 15 mM HEPES (Catalog #36254)
*Certain products are only available in select territories. Please contact your local Sales Representative or the Product & Scientific Support team at techsupport@stemcell.com for further information.
Protocol
Obtain T Cells for Organoid Co-Culture (Optional)
Obtain T cells for your co-culture experiments by isolating cells from peripheral blood mononuclear cells (PBMCs) using EasySep™ cell isolation kits. You can save time by using pre-isolated fresh or frozen PBMCs* for your experiments.
Alternatively, isolate T cells directly from whole blood using EasySep™ Direct or RosetteSep™. Explore our T Cell Isolation Products for a full list of T cell isolation kits.
The steps below are general steps to obtain T cells using EasySep™ cell isolation kits:
- Obtain PBMCs from Human Whole Peripheral Blood* using SepMate™ (see example of a SepMate™ isolation protocol). Alternatively, process Human Peripheral Blood Leukopak, Fresh* or Frozen*, for downstream isolation following the leukopak processing protocol.
- Once you have the cell suspension, follow the instructions provided in the Product Information Sheet of the particular EasySep™ kit you are using.
- The highly purified T cells can be cryopreserved for later use or expanded using ImmunoCult™-XF T Cell Expansion Medium as described in the corresponding Product Information Sheet (see Step 2.5)
Establish Organoid and T Cell Cultures and Prepare Co-Culture
- Prepare media and establish organoid cultures as described in the IntestiCult™ Product Information Sheet.
- Expand intestinal organoids as described in the IntestiCult™ Product Information Sheet.
- Prepare DMEM + 1% BSA as described in the Product Information Sheet.
- Before use in co-culture, grow organoids for 1 - 2 days after passage in IntestiCult™ Organoid Growth Medium (Human).
- Before use in co-culture, grow T cells for 1 - 2 days after passage or thawing in ImmunoCult™-XF T Cell Expansion Medium. For more information, refer to the Product Page.
- Prepare co-culture medium:
- Combine equal volumes of IntestiCult™ OGM (Complete or Basal Medium depending on whether the organoids are cancer-derived or not) and ImmunoCult™-XF T Cell Expansion Medium. Keep on ice until ready for use. Thaw an aliquot of Matrigel® on ice.
- Prior to use in the organoid culture, add 50 µL cold Matrigel® per 950 µL cold co-culture medium. Keep on ice until ready for use.
- To release and rinse the organoids from Matrigel®:
- Add 1 mL of room temperature Gentle Cell Dissociation Reagent (GCDR) on top of the exposed dome in each well.
- Incubate for 1 minute at room temperature.
- Pre-wet a 1 mL pipette tip with GCDR; use this pipette tip to thoroughly scrape the Matrigel® dome free of the well floor.
- Gently pipette the GCDR in the well up and down 2 - 3 times to break up the dome and the organoids. Ensure all pieces of Matrigel® have been rinsed free of the plate.
- Using the same pipette tip, transfer the organoid mixture to a 15 mL conical tube.
- Add 1 mL of GCDR to the newly emptied well. Using a pipette tip pre-wetted with GCDR, pipette the GCDR up and down 2 - 3 times to rinse the well. Transfer the contents of the well to the 15 mL conical tube from Step e.
- Repeat Steps a - f for each well to be used to seed co-cultures.
- Incubate the tubes at room temperature on a rocking platform set at medium speed (~40 rpm) for 10 minutes.
- Centrifuge the tubes at 290 x g for 5 minutes at 2 - 8°C. Gently pour off and discard the supernatant.
- Add 5 mL of ice-cold DMEM + 1% BSA to each tube and gently resuspend.
NOTE: When pipetting up and down, avoid touching the bottom of the well with the pipette tip. - Quantify organoids per 100 µL and calculate volume needed for 1700 organoids per 100 µL co-culture media.
- Centrifuge the tubes at 290 x g for 5 minutes at 2 - 8°C. Gently pour off and discard the supernatant.
- Resuspend organoids in co-culture media (50 µL per 1700 organoids).
- Add cultured T cells from Step 5 to organoids suspension (50,000 cells per 50 µL).
- Add 100 µL of organoid-T cell suspension to each well of a 96-well plate.
- Incubate at 37°C, 5% CO2 in a tissue culture incubator and begin co-culture timepoints.
Organoid and T Cell Co-Culture Viability Assay
- Measure the organoid viability over time (e.g. Day 0, 3, 5) using CellTiter-Glo® 3D as per the manufacturer’s instructions.
- For T cell viability measurements in different media conditions, seed 50,000 T cells per well of a 96-well plate in triplicate conditions.
- Measure T cell viability by GloCell™ Fixability Viability Dye Red 780 and flow analysis over time (e.g. Day 0, 3, 5).
Measurement of IFN-γ Secretion by ELISA
At the endpoint of co-cultures, IFN-γ can be measured by ELISA as per the manufacturer's instructions.
Measurement of Apoptosis
At specified time points during the co-cultures, Caspase-3/7 can be measured to detect cells undergoing apoptosis as per the manufacturer’s protocols.
Evaluation of IntestiCult™ and ImmunoCult™-XF Co-Culture Model for Paired CRC Patient-Derived Organoids and Tumor-Infiltrating Lymphocytes
Co-culture assays were performed using tumor-infiltrating lymphocytes (TILs) paired to CRC-patient derived organoids (PDOs) to evaluate the capacity of blended IntestiCult™ and ImmunoCult™-XF media to allow T cell reactivity under physiological conditions while also supporting organoid culture. Although undefined, TILs’ reactivity to CRC-PDOs is most likely directed to neoantigens that originate from somatic mutations in the tumor DNA. In this model, tumor reactivity relies on endogenously processed and presented peptide concentration rather than exogenous peptide-loaded PDO models. In this study, equal volumes of IntestiCult™ and ImmunoCult™-XF media (50:50) were compared with both 100% IntestiCult™ Medium (0% ImmunoCult™-XF) and 100% ImmunoCult™-XF Medium (0% IntestiCult™).
Prior to evaluating the co-culture model to determine if the blended media composition allows T cell reactivity, Caspase-3/7 apoptotic signals were measured in control conditions using 100% ImmunoCult™-XF Medium on the co-culture of CRC-PDOs and TILs, 100% ImmunoCult™-XF on an Organoid Only culture, and a positive control condition achieved using Staurosporine, an inducer of apoptosis. There was a clear development of Caspase-3/7 signals observed in the early stages of the co-culture assay with TILs in both the Staurosporine condition and the co-culture condition using 100% ImmunoCult™ Medium (Figure 1). The Organoid Only control condition successfully demonstrated negligible Caspase-3/7 due to the lack of apoptotic triggers (either T cells or Staurosporine).
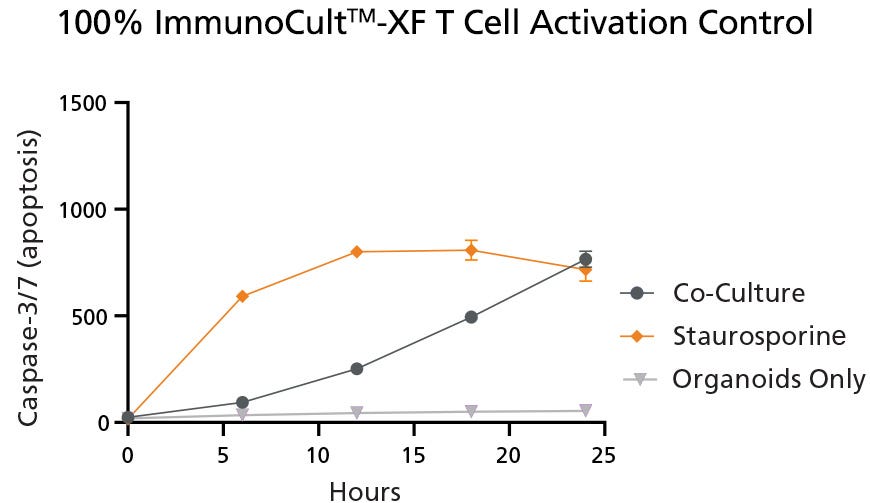
Figure 1. Quantification of Caspase-3/7 Apoptotic Signals in Control Conditions
Apoptotic signals, as measured by the level of Caspase-3/7, were present in both the positive control (Staurosporine) and Co-Culture conditions when cultured in 100% ImmunoCult™-XF. Negligible Caspase-3/7 was found in the Organoid Only condition, due to the lack of apoptotic activators. The Co-Culture condition represents CRC-PDOs and TILs co-cultured in 100% ImmunoCult™-XF Medium, the Staurosporine condition represents the co-culture cultured in 100% ImmunoCult™-XF Medium with the addition of the apoptosis inducer Staurosporine, and the Organoids Only condition represents an organoid-only culture in 100% ImmunoCult™-XF Medium. Values were presented as mean ± SD. Data used with permission from HUB Organoids.
Evaluation of the capacity of the media composition 50% IntestiCult™ and 50% ImmunoCult™-XF (referred to as 50:50) in the co-culture model with CRC-PDOs and TILs was carried out to determine if T cell activation was supported. The quantification of Caspase-3/7 apoptotic signals was used to evaluate the efficacy of the T cells to target and trigger apoptosis in the organoids. This was successfully demonstrated in Figure 2 using co-culture with the 50% blended IntestiCult™ and ImmunoCult™-XF Medium resulting in the highest levels of Caspase-3/7.
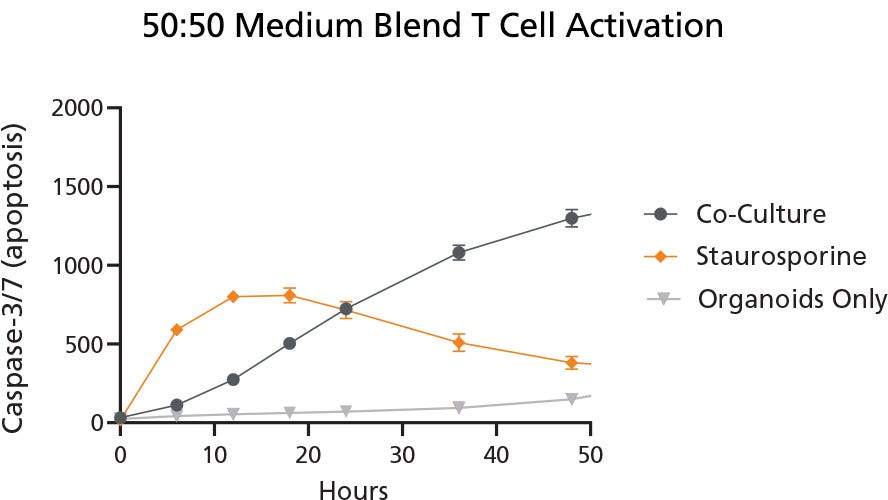
Figure 2. Quantification of Caspase-3/7 Apoptosis Signals in Blended Medium Indicated Successful T Cell Activation in Organoid Co-Culture
Apoptotic signals, as measured by Caspase-3/7, in 50:50 blended IntestiCult™ and ImmunoCult™-XF Medium indicated successful T cell activation during co-culture with CRC-PDOs. The Co-Culture condition represents CRC-PDOs and TILs co-cultured in the 50:50 medium blend, the Staurosporine condition, used as a positive control, represents CRC-PDOs and TILs co-cultured in 50:50 medium blend with the addition of the apoptotic inducer Staurosporine, and the Organoids Only condition represents CRC-PDOs cultured in the 50:50 medium blend as a negative control. Values were presented as mean ± SD. Data used with permission from HUB Organoids.
Prior to evaluating the secretion of IFN-γ under different media conditions (as a marker for T cell activity and functionality), the secretion of IFN-γ in both unactivated and activated T cells (TILs) was investigated (with Staurosporine as a control). Minimal or no IFN-γ was detected in unactivated T cell conditions, while a significant increase in secretion was observed in activated T cells (anti-CD3/CD28 antibodies) (Figure 3).
High IFN-γ production was observed in the blended medium composition of 50% IntestiCult™ and 50% ImmunoCult™ compared to 100% IntestiCult™ Medium (Figure 4).
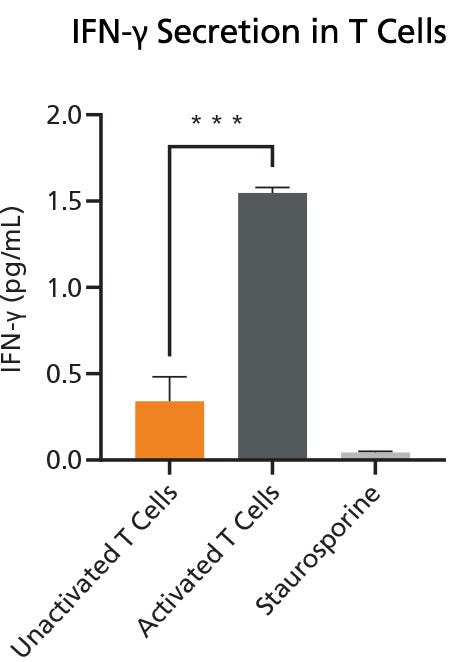
Figure 3. Quantification of IFN-γ Secretion in Activated and Unactivated T Cells
IFN-γ quantification demonstrated minimal IFN-γ in the Unactivated T cells, while a significant secretion was measured in the Activated T Cells. Values were presented as mean ± SD. P-value was calculated using unpaired two-tailed t-tests. *** p < 0.001 indicates a significant difference. Data used with permission from HUB Organoids.
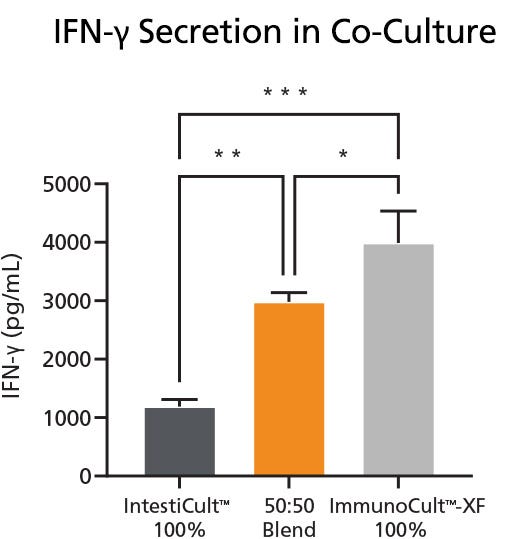
Figure 4. Quantification of IFN-γ Secretion in Activated and Unactivated T Cells
IFN-γ secretion was measured following different medium conditions for the co-culture of CRC-PDOs and TILs. IntestiCult™ 100% represents culture in 100% IntestiCult™ Medium, 50:50 Blend represents culture in a 50:50 blend of both IntestiCult™ and ImmunoCult™-XF, and ImmunoCult™ 100% represents culture in 100% ImmunoCult™-XF Medium. Values were presented as mean ± SD. P-value was calculated using one-way ANOVA with Tukey's post-hoc test for multiple comparisons. * p < 0.05, ** p < 0.01, *** p < 0.001. Data used with permission from HUB Organoids.
Conclusions
The 50:50 cell culture medium blend of 50% IntestiCult™ Organoid Growth Medium (Human) and 50% ImmunoCult™-XF T Cell Expansion Medium successfully supported patient-derived colorectal cancer organoid viability without any adverse impact on T cell viability and functionality. This technical bulletin demonstrated the capacity of using two well established STEMCELL Technologies media for co-culture applications, which opens avenues for immuno-oncology researchers exploring novel therapeutics, patient-specific therapeutic evaluation, disease mechanisms, and more.
References
- Sung H et al. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. CA Cancer J Clin. 71(3):209–49.
- Lau H.C.H. et al. (2020) Organoid Models of Gastrointestinal Cancers in Basic and Translational Research. Nat Rev Gastroenterol Hepatol 17(4):203–22.
- Fujii M et al. (2016) A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements During Tumorigenesis. Cell Stem Cell. 18(6):827–38.
CopyrightⒸ 2023 by STEMCELL Technologies Inc. All rights reserved including graphics and images. STEMCELL Technologies & Design, STEMCELL Shield Design, Scientists Helping Scientists, IntestiCult, ImmunoCult, GloCell, and EasySep are trademarks of STEMCELL Technologies Canada Inc. CellTiter-Glo is a registered trademark of Promega Corporation. Costar and Matrigel are registered trademarks of Corning Inc. All other trademarks are the property of their respective holders.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration


