Culturing Cancer-Derived Organoids Using IntestiCult™ Organoid Growth Medium (Human)
- Document # 27161
- Version 1.0.1
- Oct 2023
Cancer is one of the leading causes of death worldwide, with colorectal cancer representing one of the most common cancer types. Although cancer cell lines and animal models have revealed much information about intestinal cancers, cancer-derived intestinal organoids have been demonstrated to more faithfully recapitulate the tissue architecture, cellular heterogeneity, and morphology of the originating tumor.1 Cancer-derived organoids are consequently proving to be useful experimental models for investigating cancer biology, including disease progression and the affected signaling pathways and niche requirements of the tumor.2 Cancer-derived organoids have also enabled more translational applications, such as activating and expanding tumor-reactive T cell populations, predicting patient-specific treatment outcomes, and screening potential therapeutics.3,4
In this Technical Bulletin, we describe the culture of Wnt-independent cancer-derived organoids using IntestiCult™ OGM Human Basal Medium; Catalog #100-0190. For complete instructions, use this document in coordination with the IntestiCult™ OGM Human Basal Medium Product Information Sheet (PIS; Document #10000023716), which includes a materials list and instructions for isolating human colonic crypts from biopsy samples, establishing human intestinal organoids from the isolated crypts, as well as expanding and maintaining organoid cultures via passaging.
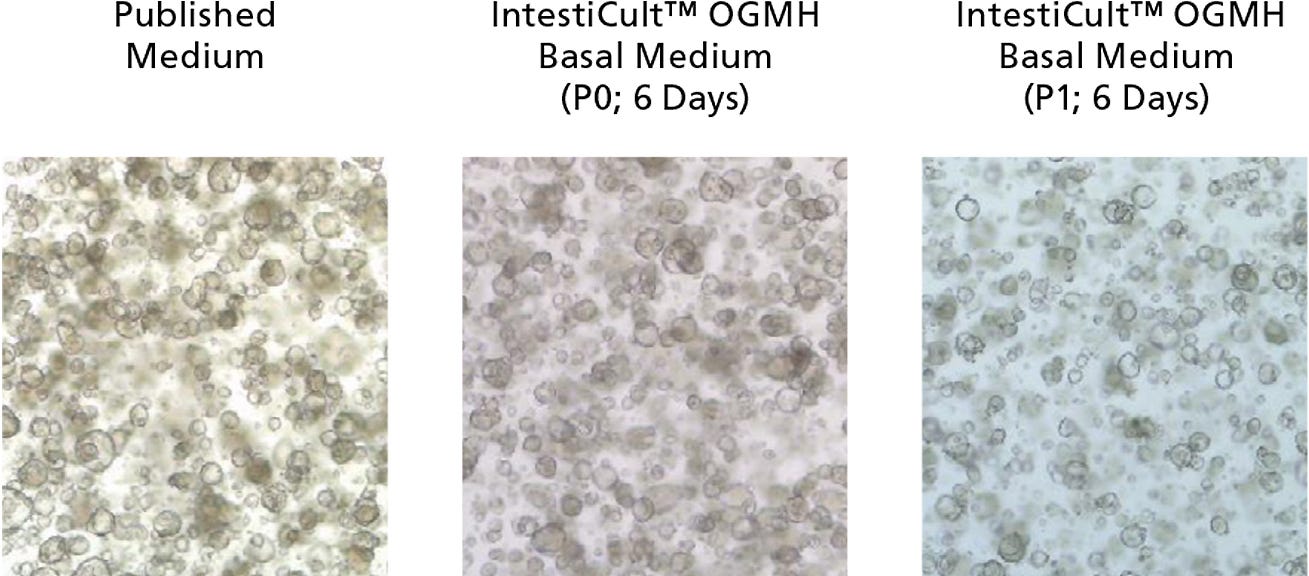
Figure 1. Growth of Cancer-Derived Organoids in IntestiCult™ OGM Human Basal Medium (OGMH)
Organoids were established from colorectal cancer biopsies in published medium,1 then switched to IntestiCult™ OGMH (P0), as described below. Cancer-derived organoids demonstrated efficient growth both after establishment in IntestiCult™ OGM Human Basal Medium, as well as after passaging. Data used with permission from HUB Organoids.
Protocol
The following protocol was developed in collaboration with Hubrecht Organoid Technology for the culture of Wnt-independent colorectal tumor biopsies in IntestiCult™ OGM Human Basal Medium. Tumors that do not carry an activating mutation of the Wnt pathway cannot be maintained as organoids using this method.
A. Medium Preparation
The following example is for preparing 100 mL of complete IntestiCult™ OGMH Medium for culture of Wnt-independent cancer-derived organoids (IntestiCult™ OGM Human Basal Medium + DMEM/F-12 with 15 mM HEPES; contact Customer Service to purchase IntestiCult™ OGMH Component A only). If preparing other volumes, adjust accordingly.
- Thaw IntestiCult™ OGM Human Basal Medium at room temperature (15 - 25°C) or at 2 - 8°C overnight. Mix thoroughly. Use immediately or aliquot and store at -20°C for up to 3 months. After thawing aliquots, use immediately. Do not re-freeze.
- Add 50 mL IntestiCult™ OGM Human Basal Medium to 50 mL DMEM/F-12 with 15 mM HEPES. Mix thoroughly. If not used immediately, store at 2 - 8°C for up to 1 week.
- Add desired antibiotics immediately before use (e.g. 50 μg/mL gentamicin).
B. Isolation of Tissue from Tumor Biopsies
- Thaw 100 μL of Matrigel® on ice.
NOTE: This is sufficient for plating up to 4 culture domes. - Place the following reagents on ice: D-PBS (Without Ca++ and Mg++) and DMEM + 1% BSA (See PIS; Document #10000023716 for preparation).
- Warm a tissue culture-treated 24-well plate in a 37°C incubator for at least 2 hours.
- In a 15 mL conical tube, wash the tissue sample with 10 mL of icecold PBS. Allow the tissue to settle by gravity (~5 seconds) then aspirate the supernatant.
- Repeat step 4, leaving 1 mL of supernatant in the tube.
- Transfer the tissue and remaining supernatant to a 1.5 mL microcentrifuge tube using a 1 mL pipettor.
- Using sterile scissors, thoroughly mince the tissue into ~5 mm pieces. Transfer the tissue fragments to a new 15 mL conical tube using a 1 mL pipettor. Rinse the microcentrifuge tube with PBS and add the rinse to the tissue fragments.
- Allow the tissue fragments to settle by gravity (~5 seconds) then aspirate the supernatant.
- Add 10 mL of Gentle Cell Dissociation Reagent. Incubate at 37°C on a rocking platform set at medium speed (~40 rpm) for 60 minutes.
- Centrifuge at 290 x g for 5 minutes. Aspirate the supernatant.
NOTE: For the remainder of the protocol, pre-wet pipette tips with DMEM + 1% BSA before manipulating the tissue sample. This prevents crypts from sticking to the wall of the pipette tip. - Add 1 mL of ice-cold DMEM + 1% BSA. Vigorously pipette up and down 20 times with a 1 mL pipettor.
NOTE: Avoid touching the tube with the pipette tip. - Using a 1 mL pipettor, pass the contents of the tube through a 70 μm cell strainer tilted on its side into a new 15 mL conical tube. Rinse the original tube with 1 mL of DMEM + 1% BSA and pass through the strainer into the tube.
C. Organoid Culture From Isolated Biopsy Tissue
For complete instructions on plating isolated tissue as organoid cultures,
proceed to section B, step 2 in the PIS (Document #100000237).
Growth medium should be replaced every 2 days, and cultures can be
passaged every 6 - 12 days. For complete passaging instructions, refer to
section C in the IntestiCult™ Organoid Growth Medium (Human) PIS (Document #10000003510). When passaging organoids
derived from cancerous tissue, use the medium prepared in section A
(above) instead of complete IntestiCult™ OGMH.
Product Information
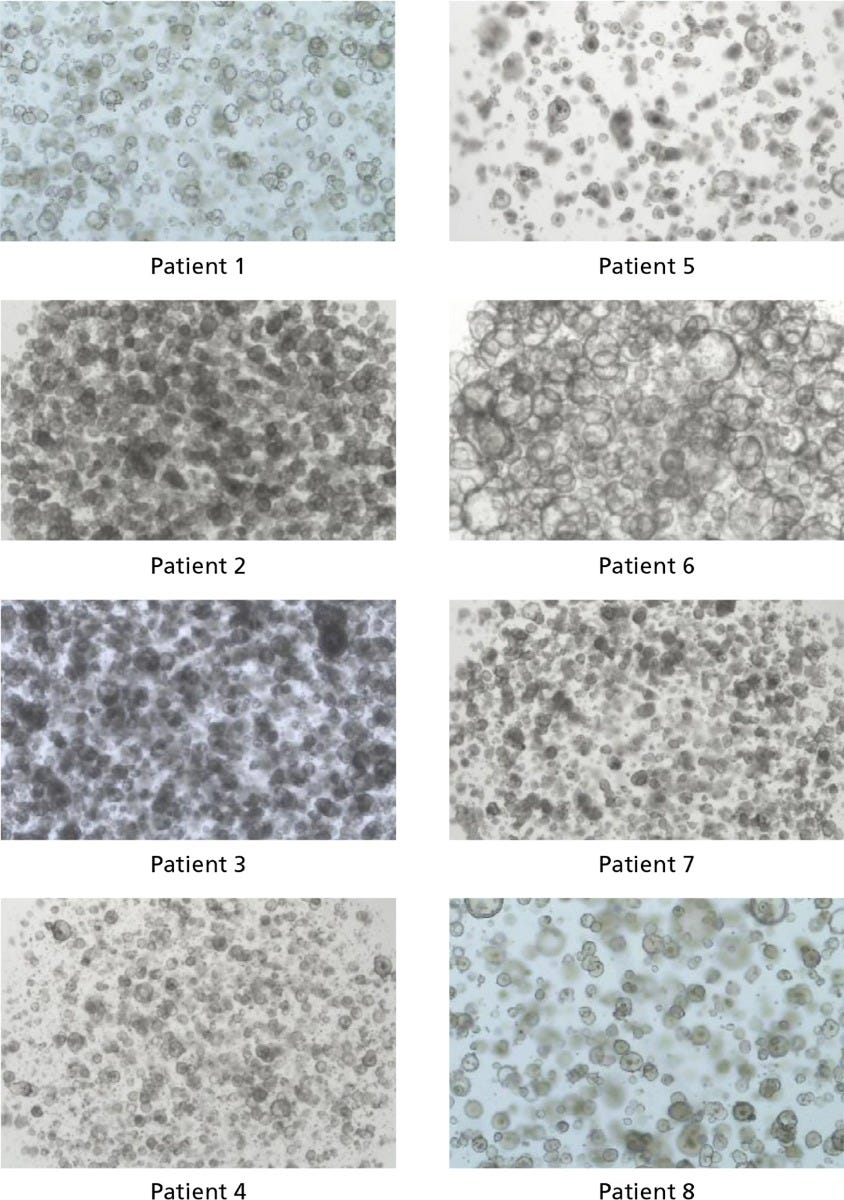
Figure 2. IntestiCult™ OGM Human Basal Medium Enables Organoid Growth Across Different Patients
Organoids were established in published medium1 from colorectal tumor biopsy samples, then switched to IntestiCult™ OGM Human Basal Medium after passaging. Organoids were passaged twice in IntestiCult™ OGMH Basal Medium and imaged at the end of the second passage (day 6 - 12). Data used with permission from HUB Organoids.
- Sato T et al. (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 141(5): 1762–72.
- Fujii M et al. (2016) A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell. 18(6): 827–38.
- Dijkstra K et al. (2018) Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 174(6): 1586–98.
- Vlachogiannis G et al. (2018) Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 359(6378): 920–26.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration


