Immune Tolerance: Central and Peripheral Tolerance
Immunology Feature
In 2003, the role of FOXP3 in regulatory T cells (Tregs) was described as a ‘master transcription factor’. Since then, the number of research studies focusing on the immunosuppressive nature of Tregs has significantly grown. FOXP3 remains the most reliable marker of Tregs to date, and the importance of Tregs in maintaining immune tolerance continues to be highlighted.
Immune tolerance is critical to prevent the development of autoimmune and inflammatory diseases. There are several mechanisms of tolerance, which can be broadly categorized as either central or peripheral tolerance. Central tolerance prevents the maturation and egress of autoreactive immune cells, for example via clonal deletion of T cells in the thymus1. Any autoreactive cells that escape central tolerance and migrate to the periphery would then encounter mechanisms of peripheral tolerance, for example the induction of anergy or suppression by mechanisms of action of regulatory T cells2 (Figure 1). Although the concept of central and peripheral tolerance has long been established, researchers continue to uncover previously unknown mechanisms of immune tolerance.
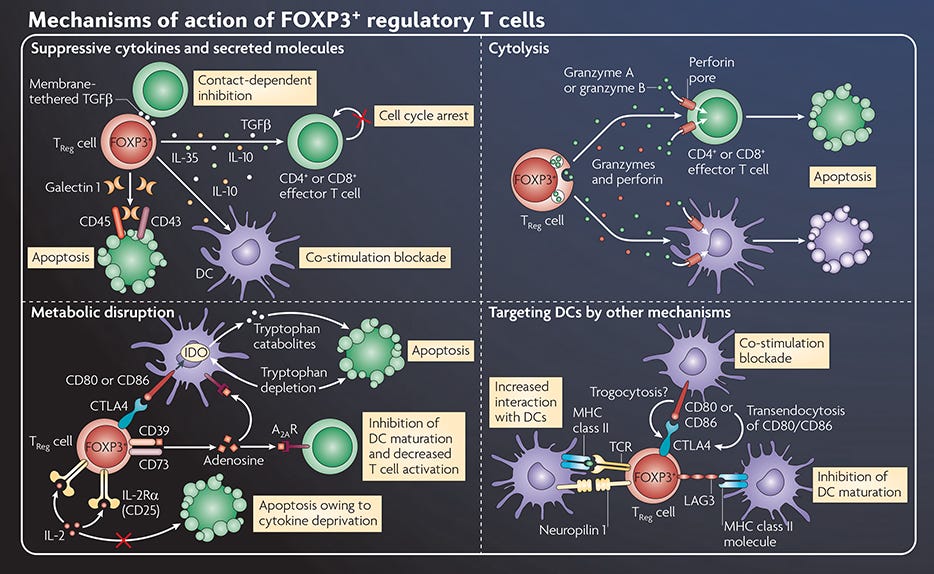
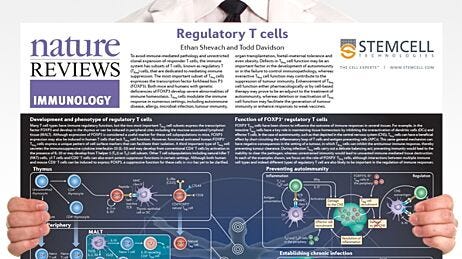
Figure 1. Mechanisms of Action of FOXP3+ Regulatory T Cells
Adapted from the Nature Reviews Immunology Regulatory T Cell wallchart, created in partnership with STEMCELL Technologies. Request a copy of the full Regulatory T Cell poster for your lab.
Central Tolerance
Multiple mechanisms of central tolerance take place in the thymus, where self-reactive T cells undergo clonal deletion or differentiation into regulatory T cells (Tregs)1.
Tolerance is established in polyclonal CD4+ T cells by distinct mechanisms, according to self-peptide expression patterns3
Nature Immunology
Self-peptides have different expression patterns in the thymus compared to the rest of the body. For example, tissue-restricted peptides may only be sparsely expressed in the thymus. How thymic self-peptide expression patterns can dictate these mechanisms of tolerance had previously been unclear. In this article, the Jenkins lab reported that T cells specific for peptides expressed uniformly in the thymus undergo deletion, whereas T cells recognizing nuclear and cytosolic proteins that are only expressed outside the thymus are retained in a state of ignorance. On the other hand, tissue-restricted peptides with limited thymic expression induced partial clonal deletion but enhanced Treg development among the remaining cells. Overall, this study shows that CD4+ T cells tolerate self peptides with different expression patterns via distinct mechanisms.
Cell separation: EasySep™ Biotin Positive Selection Kit and EasySep™ APC Positive Selection Kit
Peripheral Tolerance
Although most autoreactive T cells are deleted or converted into Tregs during their development in the thymus, some self-reactive cells will escape these mechanisms of central tolerance. Hence, multiple mechanisms of peripheral tolerance, including the ones listed below, are in place to prevent these self-reactive cells from causing damage in the periphery.
Recent thymic emigrants are tolerized in the absence of inflammation4
Journal of Experimental Medicine
For reasons that are not yet clear, the complete maturation of T cells requires a post-thymic maturation period of approximately three weeks in the periphery5. Recent thymic emigrants (RTEs) differ in TCR repertoire, proliferative responses and immunocompetence compared to mature naïve T cells. In this article, the Fink lab reported that RTEs are also more susceptible to suppression by Tregs and to anergy upon exposure to tissue-restricted antigens in the absence of inflammation, resulting in reduced proliferation and cytokine production. The authors suggest that these mechanisms may play a role in tolerance induction during post-thymic maturation; however, in the presence of inflammation, tolerance-prone RTEs retain the ability to convert into fully competent effector T cells.
Cell separation: EasySep™ Mouse CD4+ T Cell Isolation Kit and EasySep™ Mouse CD8+ T Cell Isolation Kit
Extrathymic Aire-expressing cells are a distinct bone marrow-derived population that induce functional inactivation of CD4+ T cells6
Immunity
Autoimmune regulator (Aire) is known as a transcription factor that promotes the expression of tissue-specific antigens in medullary thymic epithelial cells (mTECs). The expression of Aire in the thymus is critical for both central tolerance by clonal deletion, and for peripheral tolerance via the generation of Tregs7. Interestingly, Aire expression can also be found in extrathymic Aire-expressing cells (eTACs) in peripheral lymphoid organs. In this paper, Gardner et al. described that eTACs are a subset of antigen-presenting cells that can directly inactivate effector T cells independently of Tregs. Hence, eTACs represent another mechanism of peripheral tolerance against autoimmunity.
Cell separation: EasySep™ Mouse CD4+ T Cell Isolation Kit
The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis8
Science
Tregs are a key component of peripheral tolerance, whose role is to limit inflammation in order to prevent autoimmune and inflammatory diseases, including colitis and Crohn’s disease in the intestine. The gut is colonized with trillions of harmless commensal bacteria, collectively called the microbiota, and Tregs are critical in suppressing unnecessary immune responses against them. Interestingly, microbiota-derived signals are required for the development of intestinal Tregs. In this article, Smith et al. reported that short-chain fatty acids (SCFAs) produced by bacterial fermentation, such as propionate, acetate and butyrate, promote the generation of colonic Tregs. Furthermore, the oral administration of SCFAs can ameliorate colitis in a Treg-dependent manner in mice, emphasizing the importance of microbiota in maintaining immune tolerance in the gut.
Cell separation: Dispase and HEPES
DC-SIGN+ macrophages control the induction of transplantation tolerance9
Immunity
Tregs are not the only immunoregulatory subset of immune cells. Here, work from the Gordon lab described DC-SIGN+ macrophages as a subset of tolerogenic innate immune cells that secrete the anti-inflammatory cytokine IL-10 to suppress CD8+ T cells and promote Treg expansion. Conde et al. demonstrated that these regulatory macrophages accumulate during tolerance induction, specifically in the context of CD40L blockade in a mouse model of cardiac transplantation.
Cell separation: EasySep Mouse CD11c Positive Selection Kit
Since the role of FOXP3 in Tregs was first discovered in 200310, researchers have been working to develop Tregs as a cellular therapy to restore immune tolerance in autoimmune and inflammatory diseases, such as diabetes11. Ongoing clinical trials to test this form of therapy will reveal whether cellular therapy is an effective approach to restore immune tolerance in patients.
Explore More Immunology Features
Resources for Your Immune Tolerance Research
References
- Hogquist KA et al. (2005) Nat Rev Immunol 5: 772-782.
- Vignali DAA et al. (2008) Nat Rev Immunol 8(7): 523-532.
- Malhotra D et al. (2016) Nat Immunol 17(2): 187-195.
- Friesen TJ et al. (2016) J Exp Med 213(6): 913-920.
- Fink PJ. (2013) Annu Rev Immunol 31: 31-50.
- Gardner JM et al. (2013) Immunity 39(3): 560-572.
- Anderson MS et al. (2016) Nat Rev Immunol 16: 247-258.
- Smith PM et al. (2013) Science 341(6145): 569-573.
- Conde P et al. (2015) Immunity 42(6): 1143-1158.
- Ramsdell F et al. (2014) Nat Rev Immunol 14: 343-349.
- Bluestone JA et al. (2015) Sci Transl Med 7(315): 315ra189.