Obesity-Associated Inflammation
Immunology Feature
Although the role of pro-inflammatory cytokines in obesity-associated metabolic dysfunction had previously been described, scientists first began to identify immune cells in the adipose tissue and investigate how these cells contribute to obesity-associated inflammation in 20031.
Obesity is associated with a number of metabolic disorders, including insulin resistance. In 1993, a seminal study by Hotamisligil suggested that the pro-inflammatory cytokine TNF-ɑ contributes to insulin resistance2. Since then, many studies have convincingly shown that chronic inflammation driven by immune cells such as macrophages can contribute to the metabolic dysfunction associated with obesity.
The Accumulation of Adipose Tissue Macrophages in Obesity
Along with expanding fat mass in obesity, adipose tissue becomes heavily infiltrated with pro-inflammatory macrophages3. However, why and how macrophage-driven adipose tissue inflammation develops remains unclear. In the following articles, the Olefsky lab found that both hypoxia and leukotriene B4 are involved in promoting macrophage infiltration and adipose tissue inflammation.
Increased adipocyte O2 consumption triggers HIF-1ɑ, causing inflammation and insulin resistance in obesity4
Cell
Here, the Olefsky lab described a mechanism by which obesity-associated inflammation is promoted in response to a high-fat diet (HFD). The increase of saturated fatty acids in adipose tissue from a HFD causes an adenine nucleotide translocase 2 (ANT2)-dependent mitochondrial dysfunction in adipocytes, leading to increased oxygen consumption and causing a state of hypoxia (lack of oxygen) in the adipose tissue. In turn, this results in the activation of hypoxia inducible factor 1ɑ (HIF-1ɑ) and the secretion of chemokines by adipocytes, including monocyte chemoattractant protein-1 (MCP-1), which leads to increased adipose tissue macrophages and inflammation. Importantly, inhibition of ANT2 or HIF-1ɑ can alleviate insulin resistance in HFD mice, suggesting that the hypoxia-driven inflammation in obesity is reversible.
Cell separation: EasySep™ Mouse Monocyte Isolation Kit
LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes5
Nature Medicine
Leukotriene B4 (LTB4) is a pro-inflammatory lipid mediator that promotes the recruitment of macrophages, granulocytes and T cells. The Olefsky lab discovered that LTB4 not only has a role in the chronic inflammation found in obesity, but also has direct effects on insulin sensitivity. As expected, inhibitors of high-affinity LTB4 receptor (Ltb4r1) block macrophage chemotaxis and reduce adipose tissue inflammation in mice fed a high fat diet. Interestingly, LTB4 can directly induce insulin resistance in hepatocytes and myocytes, and the inhibition of Ltb4r1 improves insulin sensitivity and hepatic steatosis (the accumulation of fat in liver) in obese mice.
Cell separation: EasySep™ Mouse Monocyte Isolation Kit
Inflammation Alters Metabolism
Initial studies showed that inflammatory cytokines such as TNF-ɑ, which are predominantly produced by macrophages in the adipose tissue, can directly cause insulin resistance in adipocytes3. Since then, other researchers continue to demonstrate that inflammatory factors are tightly linked to metabolism in obesity.
Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes6
Cell
This Cell article outlines a novel mechanism by which inflammation can alter metabolic processes. With food intake, insulin is secreted to promote glucose uptake. In addition, insulin suppresses hepatic glucose production (HGP), a process whereby glycogen is broken down into glucose under low blood glucose conditions. This study by Perry et al. reported that insulin inhibits HGP by affecting lipolysis (the breakdown of lipids by adipocytes). Insulin inhibits lipolysis in white adipose tissues, resulting in decreased fatty acid flux to the liver and reduced hepatic acetyl CoA, which in turn suppresses HGP. Interestingly, inflammatory cytokines such as TNF-ɑ and IL-6 promote lipolysis. This study thus suggests that the overproduction of IL-6 by macrophages in obese adipose tissue contributes to the inability of insulin to control HGP in obesity. As a result, this study links macrophage-driven adipose tissue inflammation to hepatic insulin resistance.
Cell separation: EasySep™ PE Positive Selection Kit
Not Just Macrophages: the Role of Adipose Tissue T Cells
In addition to macrophages, the roles of other immune cells in obesity, including neutrophils7, B cells8 and regulatory T cells9, have now also been explored. The article featured below was among the first to detect T cells in adipose tissue and investigate their role in obesity.
Normalization of obesity-associated insulin resistance through immunotherapy10
Nature Medicine
Here, Winer et al. showed that the visceral adipose tissue of obese mice contains increased levels of IFN-γ-secreting CD4+ T cells and reduced proportions of regulatory T cells (Tregs). Interestingly, lymphocyte-deficient Rag1-/- mice have exacerbated metabolic dysfunction as a result of diet-induced obesity, which is reversed by the adoptive transfer of CD4+ T cells, suggesting a protective role of CD4+ T cells. Furthermore, treatments that increase the proportion of adipose tissue Tregs, such as administration of CD3-specific antibody or its F(ab’)2 fragment, can normalize obesity-associated insulin resistance. This study suggests that immunotherapeutic strategies to dampen inflammation can be effective against obesity-associated insulin resistance. Following this study, other groups have further elaborated on the role of Tregs and other cell types in obesity9,11 (Figure 1).
Cell separation: EasySep™ Mouse CD4+ T Cell Isolation Kit and EasySep™ Mouse CD8+ T Cell Isolation Kit
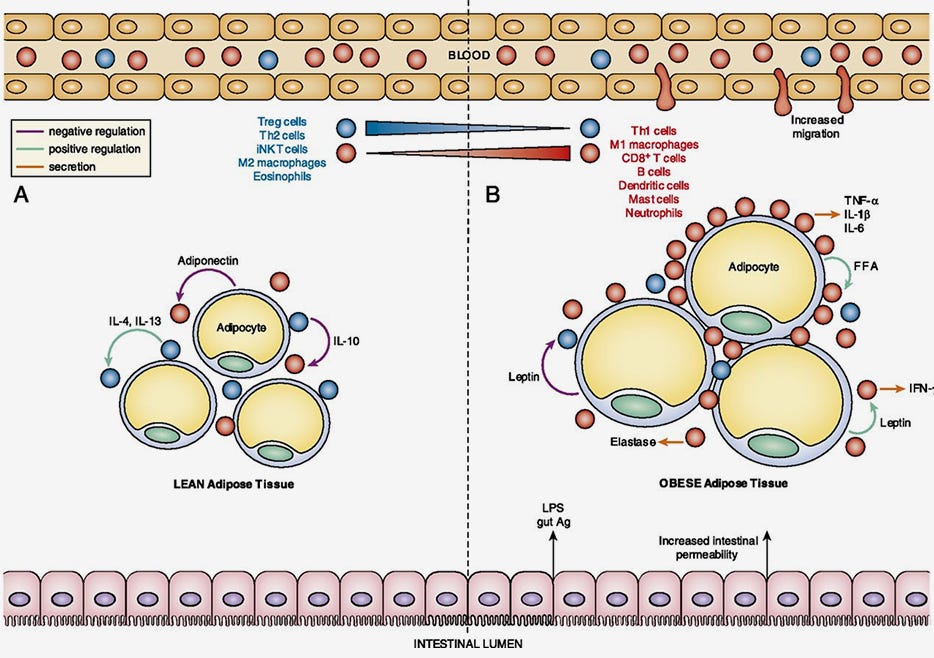
Figure 1. The Loss of Immune Regulation in Obesity-Associated Adipose Tissue Inflammation
Adapted from Han JM and Levings MK. (2013) J Immunol 191(2): 527-532. Copyright 2013. The American Association of Immunologists, Inc.
(A) Lean adipose tissue contains regulatory immune cells (blue), such as Tregs, that suppress pro-inflammatory immune cells (red). (B) In contrast, obese adipose tissue is infiltrated with pro-inflammatory immune cell, such as M1 macrophages that produce high amounts of inflammatory cytokines such as TNF-α.
Together, these studies show that inflammation plays a key role in the development of obesity-associated metabolic disorders, and suggest that strategies to treat obesity-related diseases should also therapeutically target obesity-associated inflammation. As this field of immunometabolism continues to emerge, researchers will also aim to delineate the role of inflammation in both metabolically healthy and unhealthy obese humans.
Explore More Immunology Features
Resources for Your Obesity Research
References
- Weisberg SP et al. (2003) J Clin Invest 112(12): 1796-1808.
- Hotamisligil GS et al. (1993) Science 259(5091): 87-91.
- Lumeng CN et al (2007) J Clin Invest 114: 175-184.
- Lee YS et al. (2014) Cell 157(6): 1339-1352.
- Li P et al. (2015) Nat Med 21(3): 239-2347.
- Perry RJ et al. (2015) Cell 160(4):745-758.
- Talukdar S et al. (2012) Nat Med 18(9): 1407-1412.
- Winer DA et al. (2011) Nat Med 17(5): 610-617.
- Han JM et al. (2013) J Immunol 191(2): 527-532.
- Winer S et al. (2009) Nat Med 15(8): 921-929.
- Mraz M et al. (2014) J Endocrinol 222(3): R113-R127.