StemSpan™-AOF
cGMP medium, for culture and expansion of human hematopoietic cells
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
Overview
StemSpan™-AOF contains only recombinant proteins and synthetic components, and does not contain serum or other human- or animal-derived components.
StemSpan™-AOF has also demonstrated a higher capacity than other commercially available media for the expansion of both CD34⁺CD90⁺CD45RA⁻ and CD34brightCD90⁺CD45RA⁻ subsets, enriched for hematopoietic stem cells: CD34+ cells from cord blood expanded in StemSpan™-AOF for 7 days demonstrated similar or higher repopulation than uncultured cells when transplanted into sub-lethally irradiated NSG mice and measured at both short- (3 week) and long-term (20 week) engraftment timepoints.
StemSpan™-AOF is recommended for the culture of CD34+ hematopoietic stem and progenitor cells or for differentiation to myeloid progenitors. For culture or differentiation to other cell types, visit the StemSpan™ Media Product Finder to find the best StemSpan™ media for your application.
StemSpan™-AOF is manufactured under relevant cGMPs, ensuring the highest quality and consistency for reproducible results. For additional quality information, visit www.STEMCELL.com/compliance.
STEMCELL TECHNOLOGIES maintains registered Drug Master Files for StemSpan™-AOF with the US Food and Drug Administration (FDA) and with the Japan Pharmaceuticals and Medical Devices Agency (PMDA). Request a Letter of Authorization (LOA) for StemSpan™-AOF's Drug Master File.
Note: The FDA Master File is in the process of being updated with the new format of StemSpan™-AOF: currently only the 500ml format (Catalog #100-0130) is referenced.
Please note, StemSpan™-AOF was originally launched as StemSpan™-ACF Without Phenol Red. This name change signifies that in addition to being animal component-free, no materials of animal or human origin are used in the manufacture of this medium or its components, to at least the secondary level of manufacturing. This medium also replaces StemSpan™-ACF (Catalog #09855).
• Recombinant human albumin
• Recombinant human insulin
• Recombinant human transferrin
• 2-Mercaptoethanol
• Supplements
Data Figures
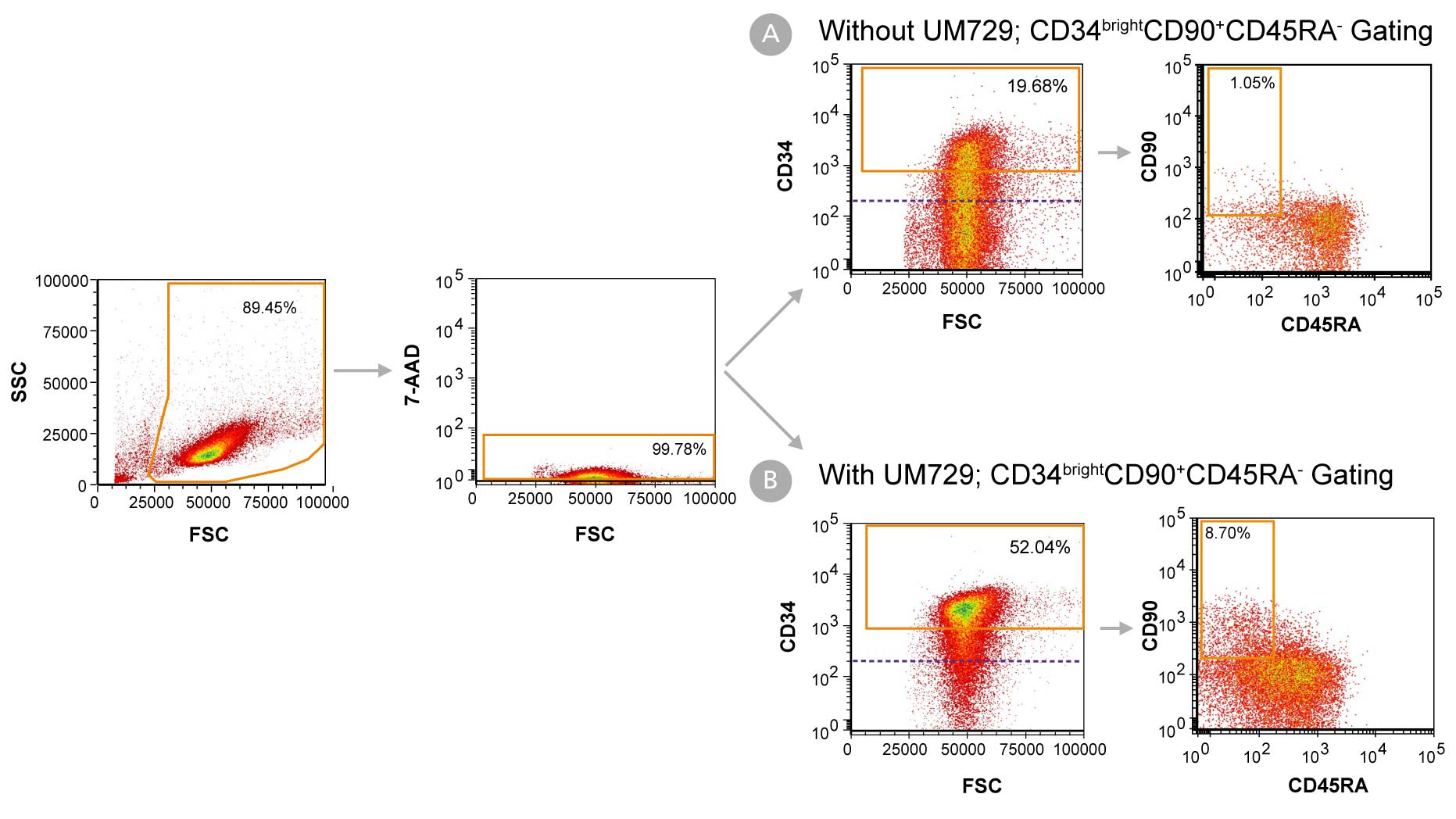
Figure 1. Day 7 Immunophenotyping of CD34+ Cells Cultured in StemSpan™-AOF
CD34+ cells were purified from cord blood (CB) using the EasySep™ Human Cord Blood CD34 Positive Selection Kit II (Catalog #17896) and cultured in StemSpan™-AOF (Catalog #100-0130) supplemented with StemSpan™ CD34+ Expansion Supplement (Catalog #02691) (A) without or (B) with the addition of UM729 (Catalog #72332). After 7 days, the cultured cells were stained with fluorescently labeled antibodies against CD34, CD90, and CD45RA, in addition to viability dye 7-AAD, and analyzed by flow cytometry. The horizontal dotted line in the CD34 vs FSC plots indicates the boundary between CD34- and CD34+ cells as based on a fluorochrome minus one (FMO) control for CD34 expression. Orange gates on these plots indicate the population of CD34bright cells used to generate data in Figures 2 and 3. Sequential gates were used to determine the percentages of viable CD34+ cells, CD34bright cells, and CD34brightCD90+CD45RA- cells.
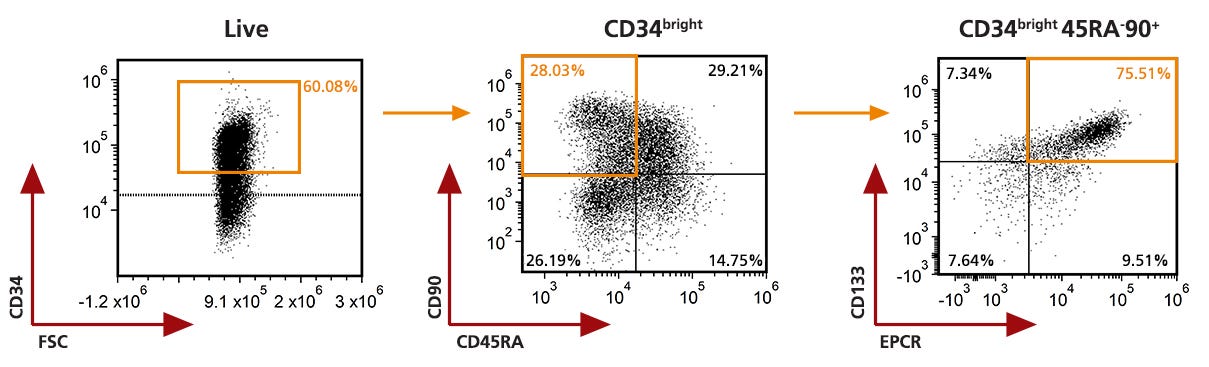
Figure 2. Analysis of the 7 days expanded Mobilized Peripheral Blood CD34+ cells by Flow Cytometry
Purified CD34+ cells derived from G-CSF mobilized peripheral blood (mPB) were cultured for 7 days in StemSpan™ AOF (Catalog #100-0130) supplemented with StemSpan™ CD34+ Expansion Supplement (Catalog #02691) and UM729 (Catalog #72332). After 7 days, the cultured cells were stained with fluorescently labeled antibodies against CD34, CD45RA, CD90, EPCR and CD133, in addition to viability dye Zombie YellowTM, and analyzed by flow cytometry. The horizontal dotted line on the CD34 and FSC plot indicated the fluorescence minus one (FMO) control for CD34 marker expression. Sequential gates (orange gates ) were used to determine the percentages of viable CD34bright cells, CD34brightCD90+CD45RA- cells and CD34bright CD45RA-CD90+CD133+EPCR+ cells and used to generate data in Figures 3.
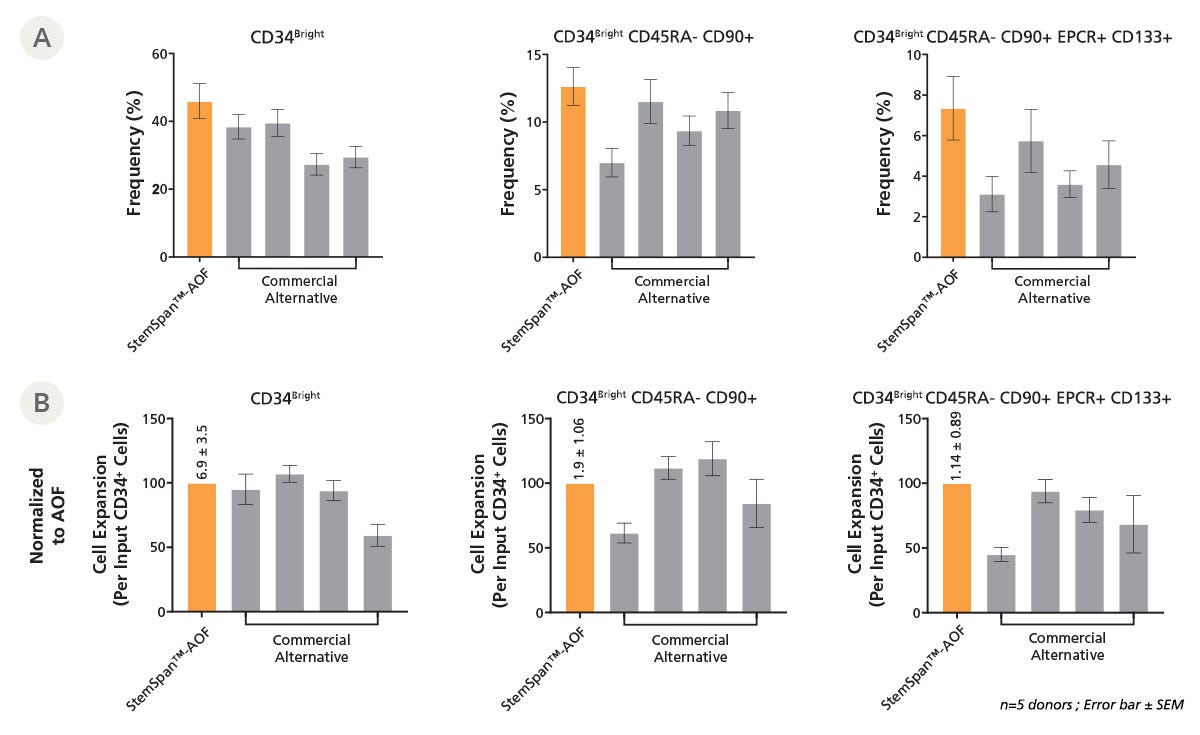
Figure 3. StemSpan™-AOF Supports Equal or Greater Expansion of Mobilized Peripheral Blood HSPCs Compared to Other Commercial Media
Purified CD34+ cells derived from G-CSF mobilized peripheral blood (mPB) were cultured at a concentration of 10000 cells per mL in StemSpan™-AOF medium (orange bars) or in four xenofree media from other suppliers (Commercial Alternatives, grey bars). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM729 (1uM). After 7 days of culture, the (A) frequency and (B) cell expansion of viable CD34bright, CD34bright CD45RA-CD90+, and CD34bright CD45RA-CD90+CD133+EPCR+ cells were analyzed by flow cytometry, as described in Figure 2, and fold expansion normalized to StemSpanTM-AOF (above bar expansion value ± SD). The performance of StemSpan™-AOF, the only animal origin-free formulation, was similar to the performance of the xeno-free alternative media. Data shown are mean ± SEM (n=5).
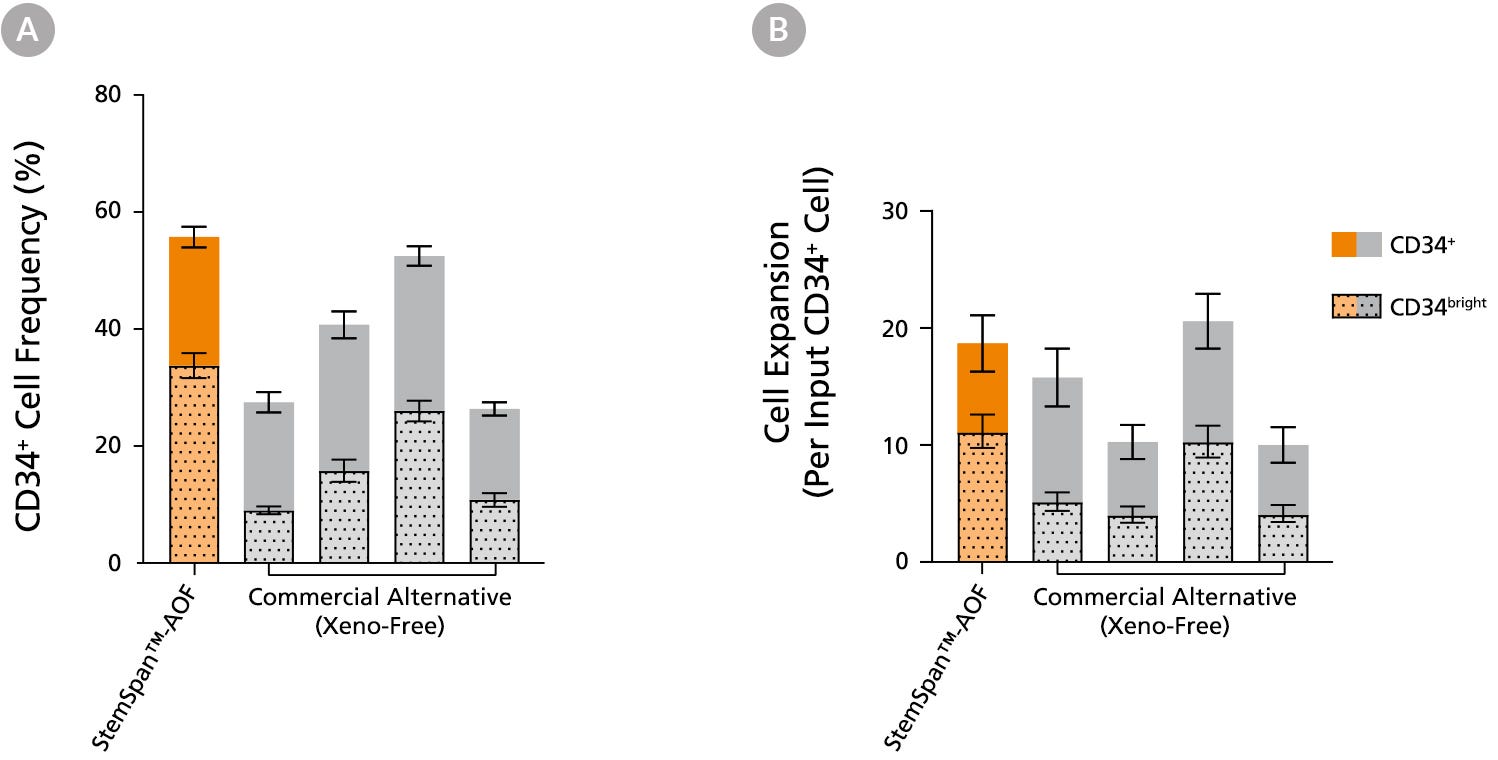
Figure 4. StemSpan™-AOF Supports Equivalent or Greater Expansion of Human CD34+ and CD34bright Cells than Other Commercial Media
Purified cord blood (CB)-derived CD34+ cells were cultured for 7 days in StemSpan™-AOF (orange bar), and in four xeno-free media formulations from other suppliers (Xeno-Free Commercial Alternative, grey bars), including (in random order) SCGM (Cellgenix), X-VIVO™ 15 (Lonza), Stemline™ II (Sigma), and StemPro™-34 (Thermo). The (A) frequency and (B) cell expansion of viable CD34+ and CD34bright cells in culture were based on viable cell counts and flow cytometry results. StemSpan™-AOF, the only animal origin-free formulation, showed equivalent performance to all xeno-free alternative media tested. All media were supplemented with StemSpan™ CD34⁺ Expansion Supplement and UM171*. Data shown are mean ± SEM (n = 8). Note: Data for StemSpan™-AOF shown were generated with the original phenol red-containing version StemSpan™-ACF (Catalog #09855). However internal testing showed that the performance of the new phenol red-free, cGMP-manufactured version, StemSpan™-AOF (Catalog #100-0130) was comparable. *Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1μM. For more information including data comparing UM171 and UM729, see Fares et al., 2014.
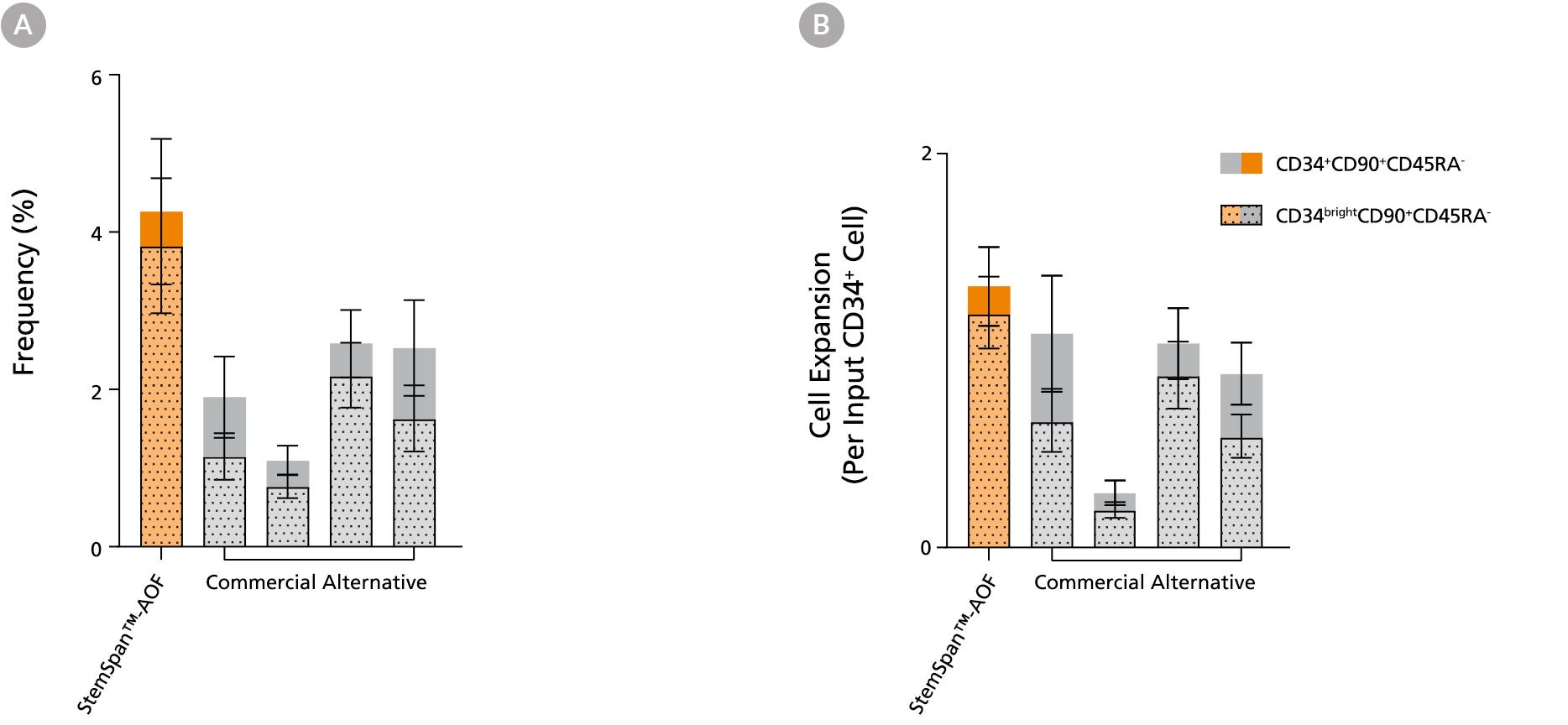
Figure 5. StemSpan™ Media Support Greater Expansion of Human CD34+CD90+CD45RA- and CD34brightCD90+CD45RA- Cells than Other Commercial Media
Purified CB-derived CD34+ cells were cultured for 7 days in StemSpan™-AOF medium (orange bar), and in four xeno-free media formulations from other suppliers (Commercial Alternative, gray bars) including (in random order) SCGM (Cellgenix), X-VIVO 15 (Lonza), Stemline II (Sigma), and StemPro 34 (Thermo). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM171*. The (A) frequency and (B) cell expansion of CD34+CD90+CD45RA- (solid) and CD34brightCD90+CD45RA-(dotted overlay) cells in culture were based on viable cell counts and flow cytometry results as shown in Figure 1. StemSpan™-AOF showed similar or significantly higher expansion of CD34brightCD90+CD45RA- cells (P Note: Data for StemSpan™-AOF shown were generated with the original phenol red-containing version StemSpan™-ACF (Catalog #09855). However internal testing showed that the performance of the new phenol red-free, cGMP-manufactured version, StemSpan™-AOF (Catalog #100-0130) was comparable. *Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1μM. For more information including data comparing UM171 and UM729, see Fares et al. 2014.
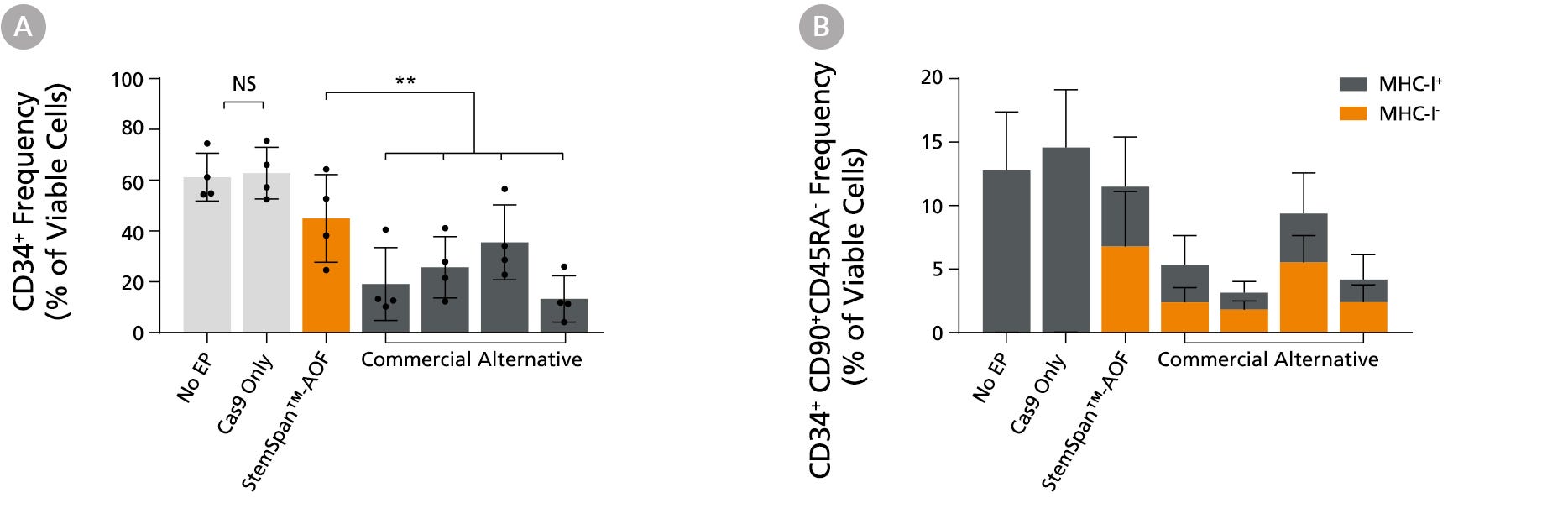
Figure 6. StemSpan™ Media Support Better CD34+ and Primitive CD34+CD90+CD45RA- HSPC Expansion in a Genome Editing Application Compared with Alternative Commercial Media
Purified CB-derived CD34+ cells were cultured for 2 days in select StemSpan™-AOF, (orange bar), or four xeno-free media formulations from other suppliers (gray bars). All media were supplemented with StemSpan™ CD34+ Expansion Supplement and UM171*. Cells were then electroporated using Arcitect™ CRISPR-Cas9 RNP complexes containing crRNA:tracrRNA targeting beta-2-microglobulin (B2M), and cultured for an additional 4 days in the same conditions. Knockout efficiency as measured by staining for MHC-I and analyzing by flow cytometry, was similar in all media tested, ~70-80%. (A) The percentage of CD34+ cells and (B) CD34+CD90+CD45RA- cells were quantified by flow cytometry 4 days post-electroporation. Data shown are mean + SD (n = 4 donors; **P < 0.01).
Note: Data for StemSpan™-AOF shown were generated with the original phenol red-containing version (Catalog #09855). However internal testing showed that the performance of the new phenol red-free, cGMP-manufactured version of StemSpan™-AOF (Catalog #100-0130) was comparable.
*Similar results are expected when using UM729 (Catalog #72332) prepared to a final concentration of 1 μM. For more information including data comparing UM171 and UM729, see Fares et al., 2014.
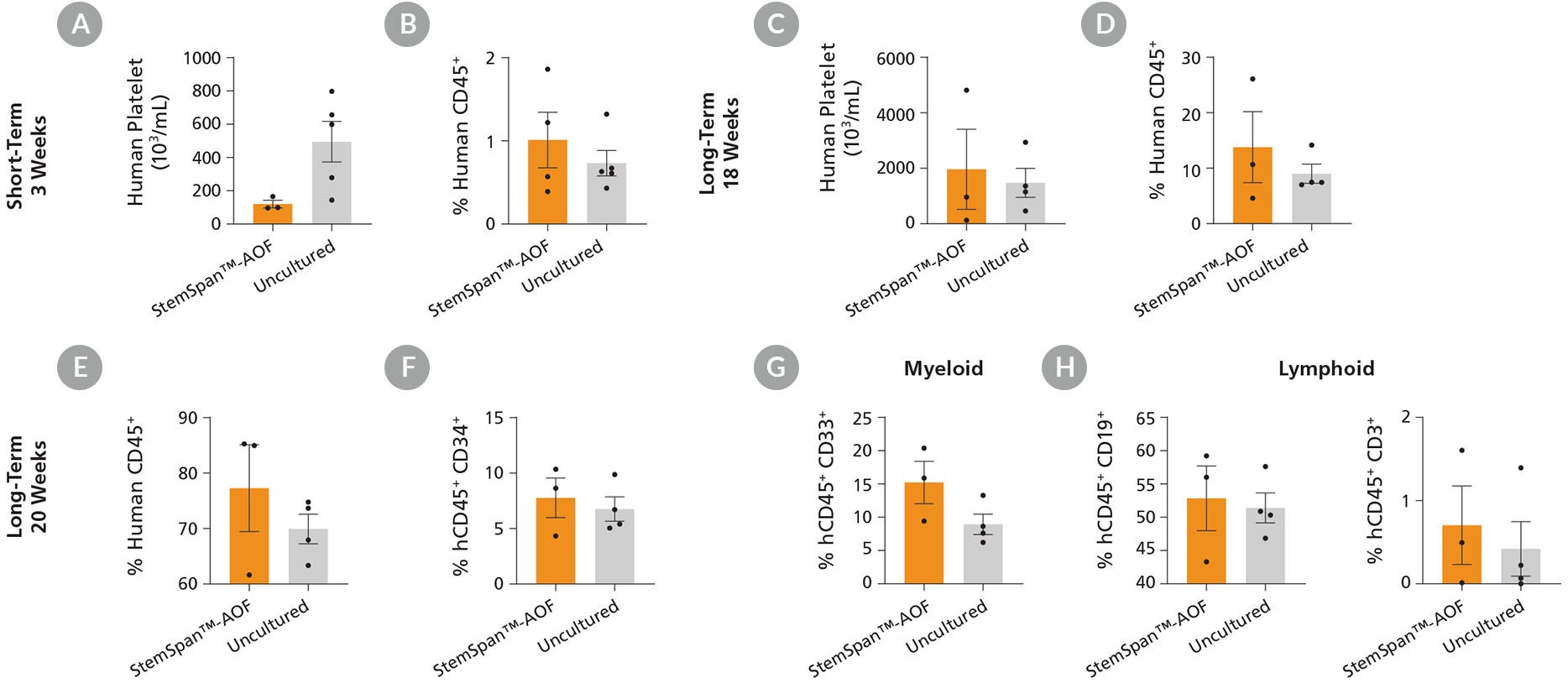
Figure 7. StemSpan™-AOF-Expanded Cord Blood CD34⁺ Cells Engraft in NSG Mouse Recipients
Purified cord blood-derived CD34⁺ cells were cultured for 7 days in StemSpan™-AOF supplemented with StemSpan™ CD34⁺ Expansion Supplement and UM729 (1 μM). After 7 days of expansion, progeny of 10,000 fresh or uncultured CD34⁺ cells were transplanted in sub-lethally irradiated NSG mice. (A-D) The number of human platelets and the frequency of human cells expressing the pan-leukocyte marker CD45 were measured in peripheral blood at 3 and 18 weeks post-transplantation. Data shown are mean ± SEM (n = 3 - 5 mice). (A) At 3 weeks, engraftment of human platelets was lower in recipients of cells cultured in StemSpan™-AOF than in recipients of uncultured cells. (C) At week 18, there were no significant differences in platelet engraftment between the expanded and uncultured cells. (B,D) Human CD45⁺ cell frequencies in recipients of cells expanded in StemSpan™-AOF were similar to those in recipients of uncultured cells. (E-H) At week 20, long-term multilineage engraftment was measured in bone marrow of transplanted NSG mice. Data shown are mean ± SEM (n = 3 - 4 mice). (E,F) Recipients of StemSpan™-AOF expanded cells showed similar frequencies of human CD45⁺ and CD34⁺ cells in the mouse bone marrow compared to recipients of uncultured cells. (G,H) Cells expanded with StemSpan™-AOF showed similar levels of myeloid (CD45⁺ CD33⁺ ) and lymphoid (CD45⁺ 19⁺ B cells and CD45⁺ CD3⁺ T cells) engraftment relative to uncultured cells.
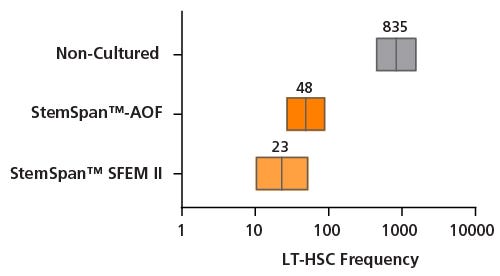
Figure 8. Estimation of Long-term SCID Repopulating Cell in Cord Blood CD34⁺ cells expanded in either StemSpan™-AOF or StemSpan™ SFEM II using Limiting Dilution Transplantation assay
Purified cord blood-derived CD34⁺ cells were cultured for 7 days in either StemSpan™-AOF or StemSpan™ SFEM II supplemented with StemSpan™ CD34⁺ Expansion Supplement and UM729 (1 μM). After 7 days of expansion, progeny of 10, 100, 250 and 2500 initial CD34⁺ cells were injected intravenously into sub-lethally irradiated NSG mice. For uncultured CD34⁺ cells, 250, 500 and 2500 cells were transplanted. The frequency of human cells expressing the pan-leukocyte marker CD45 was measured in bone marrow at ~20 weeks post-transplantation. A threshold of >0.1% of CD45⁺ cells was used to consider if mice were positive or negative for engraftment. Limiting dilution analysis was performed using the ELDA software from the Walter and Eliza Hall Institute of Medical Research software. LT-HSC frequencies (red lines) and 95% confidence intervals (boxes) are presented as 1/number of original CD34⁺ cell (day 0 equivalent) for each condition; n = 2 independent experiments performed, 2-7 mice per group per experiment. Significance level * p< 0.001 (Chi-square test). StemSpan™ SFEM II and StemSpan™-AOF expanded cells results in ~36 and ~17-fold increase in LT-HSC compared to fresh CD34⁺ cells, with SRC frequencies of 1/23, 1/48, 1/835 for StemSpan™ SFEM II, StemSpan™-AOF and fresh control respectively.
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Resources and Publications
Educational Materials (11)
Related Products
-
 StemSpan™ CD34+ Expansion Supplement (10X)
StemSpan™ CD34+ Expansion Supplement (10X)Serum-free culture supplement for expansion of human CD34+ hematopoietic cells
-
 StemSpan™ Megakaryocyte Expansion Supplemen...
StemSpan™ Megakaryocyte Expansion Supplemen...Serum-free culture supplement for expansion of human megakaryocytes
-
 MethoCult™ H4034 Optimum
MethoCult™ H4034 OptimumMethylcellulose-based medium with recombinant cytokines for human cells
-
 UM729
UM729Pyrimido-indole derivative that enhances HSC self-renewal in vitro
Item added to your cart

StemSpan™-AOF
THIS PRODUCT IS MANUFACTURED AND TESTED FOLLOWING RELEVANT CGMPs UNDER A CERTIFIED QUALITY MANAGEMENT SYSTEM. PRODUCT IS FOR FURTHER MANUFACTURING OR RESEARCH USE. NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE












