Immunomagnetic Cell Separation: Positive Selection Vs. Negative Selection
Whether to use a positive selection or negative selection approach is one of the most commonly asked questions regarding immunomagnetic cell separation.
Positive selection immunomagnetic cell separation methods involve directly labeling desired cells for selection with an antibody or ligand that targets a specific cell surface protein. The antibody or ligand is linked to a magnetic particle, allowing the labeled cells to be retained in the final isolated fraction after incubation of the sample in a magnetic field. Typical features of positive magnetic selection methods include:
- Isolated cells are highly purified
- Isolated cells are usually bound by antibodies and magnetic particles
- Antibody cocktail targets a unique surface marker on the target cells
- Additional cell populations from the negative fraction can be isolated
Negative selection immunomagnetic cell separation methods involve labeling unwanted cell types for removal with antibodies or ligands targeting specific cell surface proteins. The antibodies or ligands are linked to magnetic particles, allowing the labeled, unwanted cells to be depleted from the final isolated fraction by incubating the sample in a magnetic field. Since the desired cells are not specifically targeted by antibodies or ligands, they remain unbound by particles. Typical features of negative magnetic selection methods include:
- Isolated cells are not bound by magnetic particles
- Protocols are faster and easier with minimal sample manipulation
- Antibody cocktail target all unwanted cells and do not target desired cells
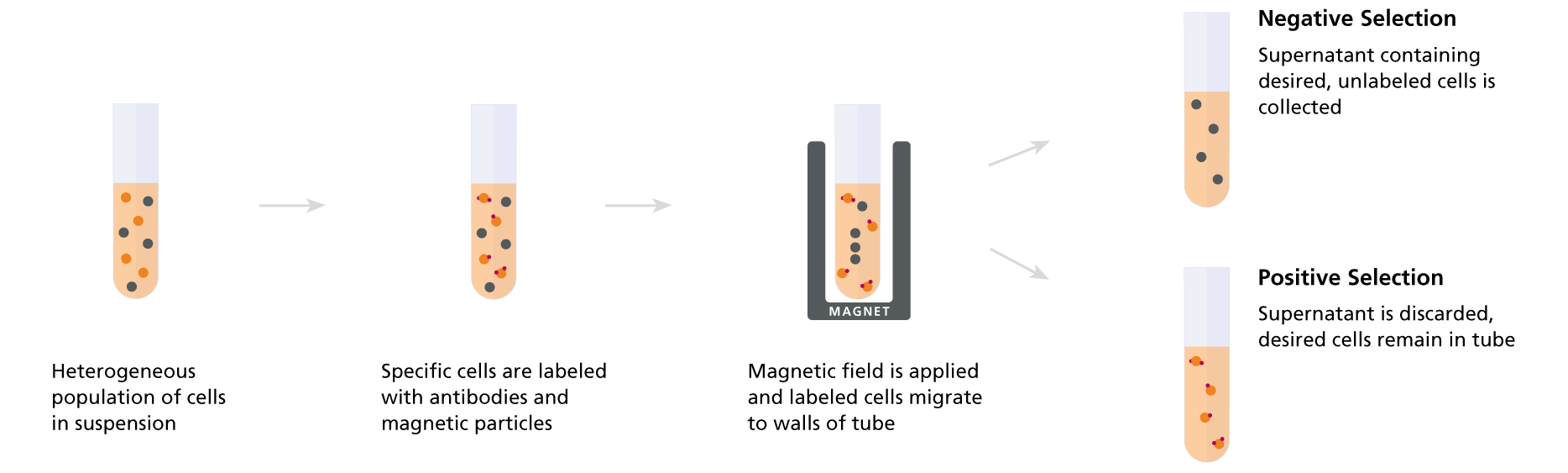
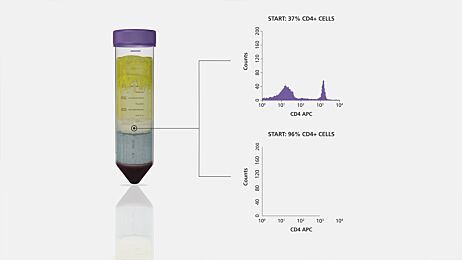
Figure 1. Comparison of Positive Selection and Negative Selection
The choice between negative and positive selection methods (Figure 1) often ultimately depends on your downstream application. For most applications, labeling cells with antibodies and EasySep™ magnetic particles during positive selection does not interfere with downstream assays. However, you should always carefully consider whether your specific research application requires unlabeled cells, which may be important when:
- Antibodies bound to a cell surface antigen is known to cause unwanted intracellular signalling
- Antibodies or magnetic particles bound to cell surface proteins affect downstream use of the cells
When in doubt, contact us to see if we have any data on or recommendations for your specific application. Below are examples of common applications of immunomagnetic cell separation and the selection method that we recommend.
Isolating rare cell types by fluorescence-activated cell sorting (FACS) may involve lengthy sort times. Researchers can substantially reduce the required cell sorting time by using immunomagnetic cell separation to pre-enrich their target cells. In this case, quick protocols and high recovery are desirable to save time and maximize target cell yield resulting from the flow sorting procedure. Negative selection is typically an ideal approach because it is quick, has high recovery, and doesn't label the cells of interest. This allows researchers to use any fluorophore-tagged antibodies against specific cell surface markers for their FACS.
Pre-enrichment can be done upstream of FACS for a wide variety of specific cell subsets, including antigen-specific cells, subsets of T helper cells, B cells, and innate lymphoid cells (ILCs).
View our fast negative selection kits that are ideal for pre-enrichment:
For the isolation of uncommon cells for which there are no specific, commercially available cell separation kits, indirect positive selection may be used. This method provides the flexibility of using your own primary antibodies to label the desired target cells. Immunomagnetic cell separation can then be achieved by using commonly available secondary antibodies to attach magnetic particles to the target cells labeled by the primary antibodies. In this way, almost any cell type may be isolated via indirect positive selection.
Learn more about isolating virtually any cell type using indirect positive selection kits >
Conveniently, some cell separation providers also offer custom cell isolation kits that can be specifically designed for a particular starting cell source, species, and virtually any desired cell type or target cell surface antigen(s). For example, custom EasySep™ kits allow you to isolate or enrich any cell type of interest.
Complex cell types may require a combination of negative and positive selection for successful purification. For example, the isolation of CD4+CD127lowCD25+ regulatory T cells (Tregs) can be challenging due to the requirement to select cells based on three different cell surface markers. Combining negative and positive selection strategies makes this possible. See the diagram below outlining the protocol of our EasySep™ Human CD4+CD127lowCD25+ Regulatory T Cell Isolation Kit.
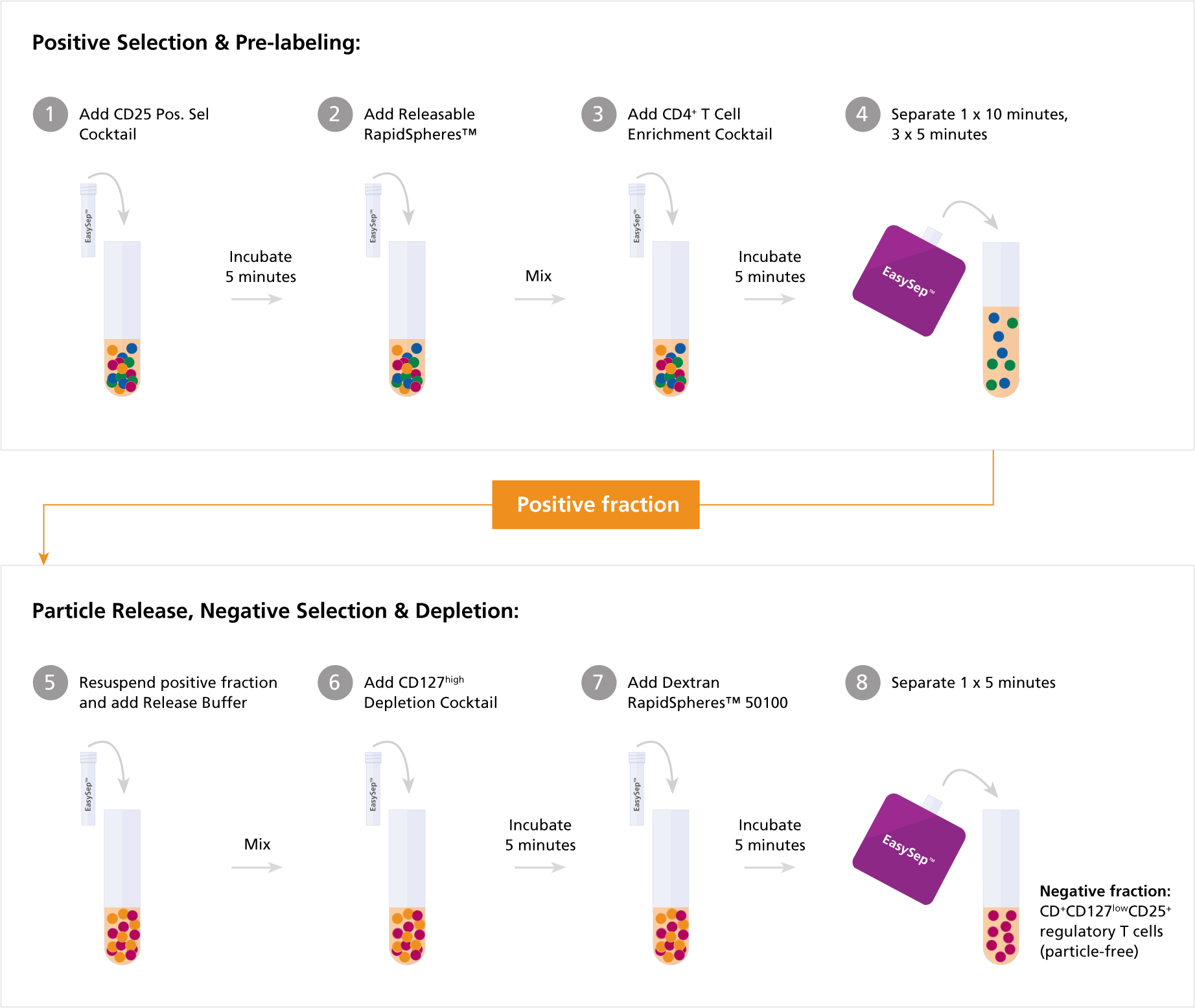
Figure 2. Protocol of Our EasySep™ Human CD4+CD127lowCD25+ Regulatory T Cell Isolation Kit
You can also isolate multiple cell types from a single sample through a sequential separation procedure. This may be particularly useful when your sample volume is limited and you do not wish to divide the sample. Learn more >
Still not sure which method to use? Compare our negative and positive selection EasySep™ kits to decide for yourself: