CRISPR-Cas9 Genome Editing of Human Intestinal Organoids
CRISPR-Cas9 genome editing of intestinal organoids cultured in IntestiCult™ Organoid Growth Medium (Human) (Catalog #06010) using the ArciTect™ CRISPR-Cas9 ribonucleoprotein (RNP)-based system and STEMCELL's Guide RNA Design Tool
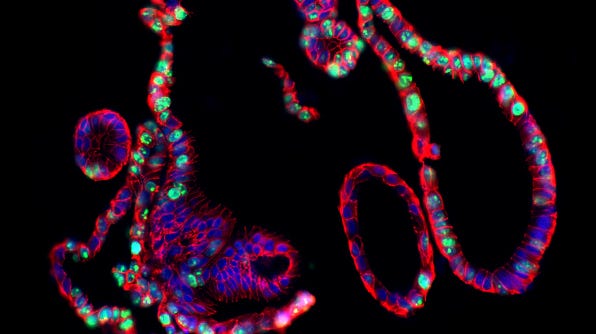
Intestinal organoids are 3D cell cultures that recapitulate the cellular identity and organization of the adult intestinal epithelium. A key benefit of intestinal organoid cultures is that they are amenable to genome editing, such as by CRISPR-Cas9, an RNA-guided technology that has revolutionized genome editing due to its ease and efficiency. Combining CRISPR-Cas9 and organoids can allow researchers to develop more physiologically relevant in vitro human disease models and study organ development.
Below, we describe a protocol for CRISPR-Cas9 genome editing of human intestinal organoids cultured in IntestiCult™ Organoid Growth Medium (Human) (Catalog #06010) using the ArciTect™ CRISPR-Cas9 ribonucleoprotein (RNP)-based system and STEMCELL’s Guide RNA Design Tool.
Materials
- ArciTect™ Cas9 Nuclease* (Catalog #76002 / 76004)
- ArciTect™ sgRNA† (Catalog #200-0013)
- ArciTect™ Human CRISPR Optimization Kit (Catalog #100-0470, 100-0471, 100-0472)
- IntestiCult™ Organoid Growth Medium (Human) (Catalog #06010)
- IntestiCult™ OGM Human Basal Medium, 50 mL (formerly IntestiCult™ OGM Human Component A)
- Organoid Supplement, 50 mL (formerly IntestiCult™ OGM Human Component B)
- DMEM/F-12 with 15 mM HEPES (Catalog #36254)
- 25% Bovine serum albumin (BSA) in phosphate-buffered saline (PBS)
- Corning® Matrigel® Matrix, Growth Factor Reduced (GFR), Phenol Red-Free (Corning 356231)
- D-PBS (Without Ca++ and Mg++) (Catalog #37350)
*ArciTect™ Cas9-eGFP Nuclease (Catalog #76006) can also be used. All testing was performed using ArciTect™ Cas9 Nuclease.
†This product is only available in Australia, Austria, Belgium, Canada, China, Denmark, Finland, France, Germany, Iceland, Ireland, Luxembourg, the Netherlands, New Zealand, Norway, Poland, Portugal, Singapore, Spain, Sweden, Switzerland, the United Kingdom, and the United States.
For information regarding culture and passaging of human intestinal organoids using IntestiCult™ Organoid Growth Medium (Human), refer to the Product Information Sheet (Document #DX21423).
The materials required are indicated on a per-well basis. These values will need to be scaled up for the actual number of wells in an experiment. Multiple guide RNA (gRNA) targeting sequences are often tested when targeting a new gene, as different gRNAs can exhibit a range of targeting/editing efficiencies at both on- and off-target sites. Single guide RNA (sgRNA) can be designed using the Guide RNA Design Tool from STEMCELL. This tool uses best practices and the latest computational tools to design optimal targeting sequences for every gene in the human and mouse genomes.
Protocol
Part I: Preparation of ArciTect™ sgRNA Working Solution
- Briefly centrifuge the vials before opening.
- Add nuclease-free water to each vial to give a final concentration of 100μM single guide RNA (sgRNA), as indicated in Table 1.
Table 1. Resuspension Volume for 100 μM* ArciTectTM sgRNA
Amount of ArciTect™ sgRNA Volume of Nuclease-free Water (µL) 4 nmol 40 *100 µM is equal to 100 pmol/µL
- Mix thoroughly. If not used immediately, aliquot and store at -20°C for up to 6 months or at -80°C for longer than 6 months. After thawing the aliquots, use immediately. Do not re-freeze.
Part II: Preparation of Compete IntestiCult™ Organoid Growth Medium (Human) and DMEM + 1% BSA
Use sterile technique to prepare complete IntestiCult™ Organoid Growth Medium (Human Basal Medium + Organoid Supplement). The following example is for preparing 100 mL of complete medium. If preparing other volumes, adjust accordingly.
- Thaw Human Basal Medium & Organoid Supplement at room temperature (15 - 25°C) or at 2 - 8°C overnight. Mix thoroughly.
Note: Once thawed, use immediately or aliquot and store at -20°C for up to 3 months. After thawing aliquots, use immediately. Do not re-freeze.
- Add 50 mL of Organoid Supplement to 50 mL of Human Basal Medium. Mix thoroughly.
Note: If not used immediately, store complete medium at 2 - 8°C for up to 1 week.
- Add desired antibiotics immediately before use (e.g. 50 µg/mL gentamicin or 100 units [100 µg/mL] penicillin/streptomycin).
Use sterile technique to prepare DMEM + 1% BSA. The following example is for preparing 50 mL of DMEM + 1% BSA. If preparing other volumes, adjust accordingly.
- Add 2 mL of 25% BSA to 48 mL DMEM/F-12 with 15mM HEPES in a 50 mL conical tube (e.g. Catalog #38010).
- Mix well by inversion. Place on ice.
Note: If not used immediately, store at 2 - 8°C for up to 6 months.
Part III: Preparation of Intestinal Organoid Single-Cell Suspension for Electroporation
- Warm a 24-well tissue culture plate(s) in a 37°C incubator for at least 2 hours prior to plating matrigel domes.
- Warm complete IntestiCult™ Organoid Growth Medium (Human) to room temperature.
Note: For each well to be plated (electroporation condition), 750 µL of medium will be required.
- Thaw Matrigel® on ice.
Note: For each well to be plated (electroporation condition), 25 µL of Matrigel® will be required.
- Place DMEM + 1% BSA on ice.
- Prepare 1 mL ACCUTASE™ + 10 µM Y-27632 per well to be collected. Warm to room temperature.
- Carefully remove and discard medium from each well to be collected for electroporation, without disturbing the Matrigel® dome.
- Add 500 µL of ACCUTASE™ + 10 µM Y-27632 on top of the exposed dome in each well. Incubate for 1 minute at room temperature.
- Pre-wet a 1 mL pipette tip with DMEM + 1% BSA; use this pipette tip to thoroughly rinse and scrape the Matrigel® dome free of the well floor. Pipette the solution up and down 2 - 3 times to break up the dome and the organoids. Ensure all pieces of Matrigel® have been rinsed free of the plate.
- Using the same pipette tip, transfer the organoid mixture to a DNase- and RNase-free microcentrifuge tube.
Note: If collecting organoids from multiple wells, transfer mixture to a 15 mL Falcon® tube (Catalog #38009).
- Add 500 µL of ACCUTASE™ + 10 µM Y-27632 to the same well and use a pre-wetted pipette tip to free any remaining pieces of Matrigel®. Transfer the organoid mixture to the same tube as above.
- Incubate the organoid mixture in a 37°C water bath or heat block for 20 minutes. Remove from incubation and pipette mixture up and down 15 - 20 times every 5 minutes to break apart Matrigel® dome and dissociate organoids into a single-cell suspension.
Note: It is helpful to check cell suspension with a phase contrast microscope to ensure single-cell suspension has been generated after the 20 minute incubation.
- Centrifuge cell suspension at 300 x g for 5 minutes.
- Remove supernatant and resuspend cell pellet in 1 mL DMEM + 1% BSA. Run suspension through cell strainer. Count cells.
- Prepare 1 x 105 cells per electroporation reaction and centrifuge at 300 x g for 5 minutes.
- Proceed immediately to part IV.
Part IV: Preparation of ArciTect™ CRISPR-Cas9 RNP Complex Mix for Electroporation
- To prepare the RNP Complex Mix, combine the components in the order listed in Table 2 (Neon® Electroporation) or Table 3 (4D-Nucleofector™ X Electroporation) in a microcentrifuge tube. Adjust component volumes according to the desired number of transfections.
Table 2. Preparation of RNP Complex Mix Using the sgRNA for Neon® Electroporation
Component Neon® Electroporation
(Volume per Reaction (μL)Resuspension Buffer T 6.00 ArciTect™ Cas9 Nuclease (4 μg/μL; 25μM)* 0.90 100 μM sgRNA 0.60 Total 7.50 Notes:- May require optimization with different cell sources. A 1:2 (shown) to 1:8 molar ratio of Cas9 to guide RNA is recommended.
- These values are for a single electroporation reaction and include pipetting error for Neon® electroporation. Scale up as necessary.
Table 3. Preparation of RNP Complex Mix Using the sgRNA for 4D-Nucleofector™ X Electroporation
Component 4D-Nucleofector™ X Electroporation
(Volume per reaction (μL)P3 Primary Cell Nucleofector™ Solution with Supplement 1 6.00 ArciTect™ Cas9 Nuclease (4 μg/μL; 25μM)* 0.90 100 μM sgRNA 0.60 Total 7.50 Notes:- May require optimization with different cell sources. A 1:2 (shown) to 1:8 molar ratio of Cas9 to guide RNA is recommended.
- These values are for a single electroporation reaction and include pipetting error for 4D-Nucleofector™ X electroporation. Scale up as necessary.
- Mix thoroughly.
- Incubate the RNP Complex Mix at room temperature (15 - 25°C) for 10 - 20 minutes.
Part V: Electroporation of Intestinal Stem Cells with RNP Complex
Perform electroporation using either the Neon® Transfection System (section a) or the Lonza® 4D-Nucleofector™ X Unit (section b).
a) Electroporation Using Neon® Transfection System
- Aspirate supernatant from the cell pellet (prepared in part III). Resuspend cells in 7.5 μL of Resuspension Buffer T per electroporation condition and pipette up and down vigorously to mix.
- Transfer 7.5 μL of the cell suspension to each 7.5 μL of RNP Complex Mix (prepared in part IV) and pipette up and down gently to mix, trying not to form air bubbles.
- Using a 10 μL Neon® pipette tip, draw up 10 μL of the mixture, check to see if the capillary is free of bubbles, and place into the electroporation chamber containing 3 mL of Electrolytic Buffer E.
Note: If air bubbles are present in the tip when the cells are electroporated, cell viability and transfection efficiency will be significantly reduced.
- Electroporate the mixture using the settings in Table 4.
Note: Refer to the manufacturer’s instructions on electroporation. Electroporation conditions may require optimization for different cell sources.
Table 4. Recommended Electroporation Conditions for Intestinal Cells Using a Neon® Transfection System
Electroporation Parameter Electrical potential 1600 V Pulse width 20 ms Number of pulses 2
b) Electroporation Using Lonza® 4D-Nucleofector™ X Unit
- Aspirate supernatant from the cell pellet (prepared in part III). Resuspend cells in 17.5 μL of P3 Primary Cell Nucleofector™ Solution + Supplement 1 per electroporation condition and pipette up and down vigorously to mix.
- Transfer 17.5 μL of the cell suspension to each 7.5 μL RNP Complex Mix (prepared in part IV) and pipette up and down gently to mix, trying not to form air bubbles.
Note: If air bubbles are present in the cuvette when the cells are electroporated, cell viability and transfection efficiency will be significantly reduced.
- Transfer 25 μL of the cell suspension + RNP Complex Mix to one well of the 16-well Nucleocuvette™ Strip. Gently tap or use a pipette tip to ensure no air bubbles are present.
- Set the Lonza® 4D-Nucleofector™ X Unit to program code DS-138.
- Place the Nucleocuvette™ Strip in the Shuttle device of the 4D-Nucleofector™ X Unit, select OK to load the strip, and select Start to begin electroporation.
Part VI: Post-Editing Culture
- Immediately after electroporation, transfer cells to a DNase- and RNase-free microcentrifuge tube. Centrifuge at 300 x g for 5 minutes.
- Remove supernatant and resuspend cell pellet in 25 μL ice-cold DMEM + 1% BSA. Pipette up and down vigorously 10 times to thoroughly resuspend single-cell suspension. Take care not to generate bubbles. Place tube on ice.
- Add 25 μL Matrigel® to the sample tube. Pipette up and down to mix. Avoid introducing bubbles. Place tube on ice until ready to plate.
- Pre-warm a 24-well tissue culture-treated plate overnight or a minimum of 1 to 2 hours.
- Using a pre-wetted pipette tip, draw up 50 μL of the Matrigel®-cell suspension and add to 1 well of a 24-well tissue culture-treated plate as follows:
a. Hold the pipette vertically over the center of the well. Bring the pipette tip near to but not in contact with the floor of the well.
b. Slightly depress the plunger until a droplet is visible on the end of the pipette tip.
c. Slowly lower the pipette until the droplet touches the floor of the well.
d. Gently dispense (only to the first stop on the pipette) the remaining volume while lifting the pipette away from the well.Note: Work quickly to plate the Matrigel®-cell suspension within ~60 seconds of removing it from ice. - Carefully transfer the plate to a 37°C incubator. Incubate for 10 minutes to allow domes to solidify. Do not disturb the domes.
- Add 750 μL of complete IntestiCult™ Organoid Growth Medium supplemented with 10 μM Y-27632 (final concentration) to each well by pipetting the medium gently down the wall of the well. Do not pipette directly onto the domes.
- Add sterile PBS to unused wells to maintain a humid environment. Place the lid on the culture plate and incubate at 37°C and 5% CO2.
- Every 2 days, perform a full-medium change with complete IntestiCult™ Organoid Growth Medium.
- Harvest cells for assessment of genome editing efficiency after 7 - 10 days of culture.
Note: Genomic DNA can be amplified by PCR using primers flanking the target region and ArciTect™ High-Fidelity DNA Polymerase Kit (Catalog #76026), followed by sequencing of the PCR products. Alternatively, ArciTect™ T7 Endonuclease I Kit (Catalog #76021) can be used to assess editing efficiency (% INDEL formation) following PCR amplification.
Data
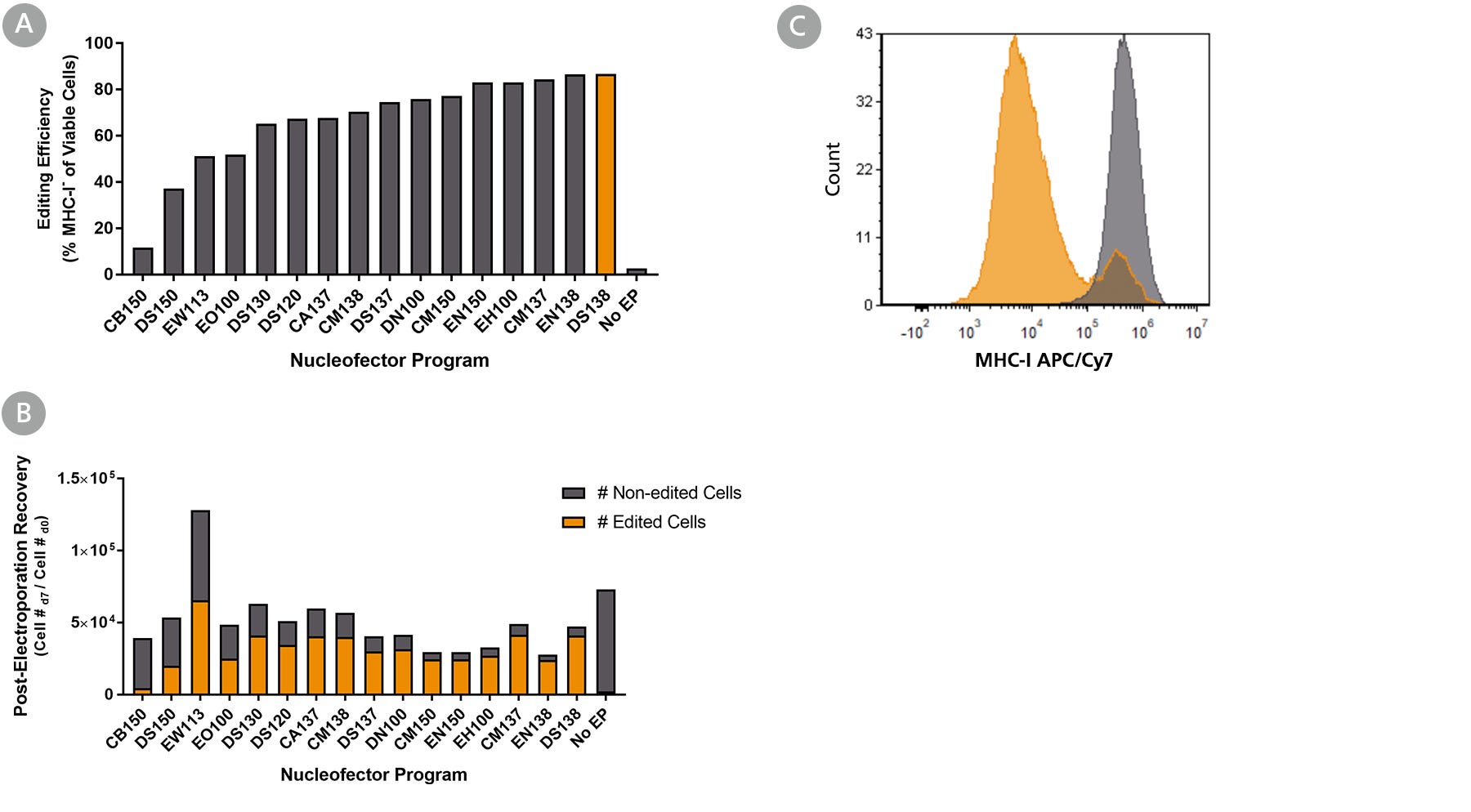
Figure 1. Optimization of Intestinal Organoid-Derived Cell RNP Delivery Using the 4D-Nucleofector™ System
Intestinal organoids were derived from a healthy control (colon biopsy sample, line A) and cultured in IntestiCult™ Organoid Growth Medium (Human) for 10 passages. RNP complexes containing ArciTect™ Cas9 Nuclease and ArciTect™ sgRNA targeting the B2M gene were delivered using the indicated 4D-Nucleofector™ program, and editing efficiency was monitored by flow cytometry to detect surface expression of major histocompatibility complex (MHC) class I molecules (MHC-I). (A) Editing efficiency was measured 7 days after electroporation by flow cytometry using the ArciTect™ Human CRISPR Optimization Kit, APC to detect functional knockout of B2M by detecting loss of MHC-I expression on the cell surface. (B) The total number of edited cells (orange bars) and non-edited intestinal organoid cells (grey bars) was calculated by multiplying editing efficiency by post-electroporation recovery; this indicated which electroporation programs resulted in best overall editing efficiency and cell recovery. (C) Representative histogram of MHC-I flow cytometry data from RNP-electroporated (RNP, orange; DS-138) and non-electroporated (No EP; grey) intestinal organoid cells 7 days after delivery of the CRISPR-Cas9 RNP complexes containing sgRNA targeting B2M.
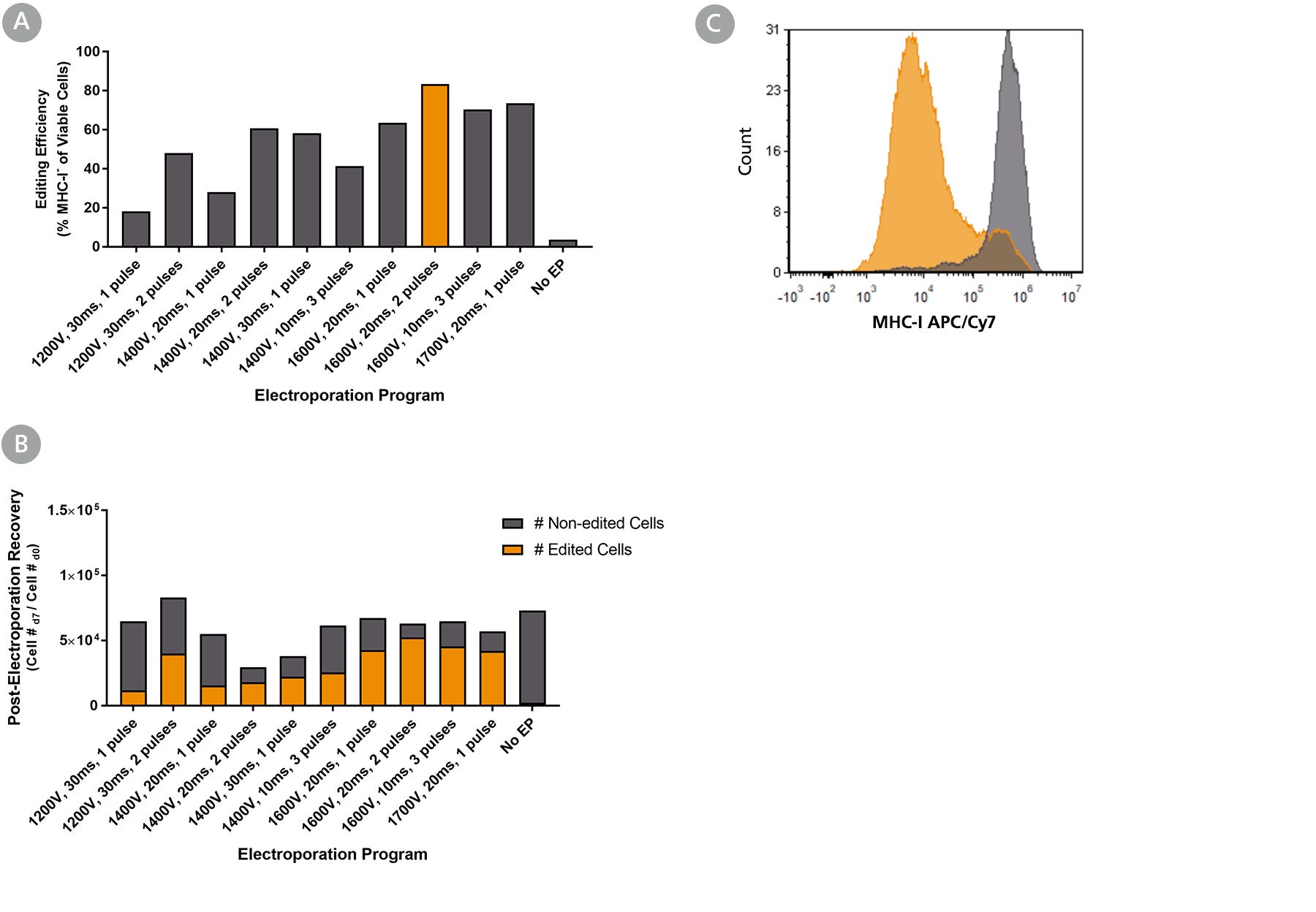
Figure 2. Optimization of Intestinal Organoid-Derived Cell RNP Delivery Using the Neon® Transfection System
Intestinal organoids were derived from a healthy control (colon biopsy sample, line A) and cultured in IntestiCult™ Organoid Growth Medium (Human) for 10 passages. RNP complexes containing ArciTect™ Cas9 Nuclease and ArciTect™ sgRNA targeting the B2M gene were delivered using the indicated Neon® Transfection System settings, and editing efficiency was monitored by flow cytometry to detect surface expression of major histocompatibility complex (MHC) class I molecules (MHC-I). (A) Editing efficiency was measured 7 days after electroporation by flow cytometry using the ArciTect™ Human CRISPR Optimization Kit, APC to detect functional knockout of B2M by detecting loss of MHC-I expression on the cell surface. (B) The total number of edited cells (orange bars) and non-edited intestinal organoid cells (grey bars) was calculated by multiplying editing efficiency by post-electroporation recovery; this indicated which electroporation programs resulted in best overall editing efficiency and cell recovery. (C) Representative histogram of MHC-I flow cytometry data from RNP-electroporated (RNP, orange; 1600 V, 20 ms, 2 pulses) and non-electroporated (No EP; grey) intestinal organoid cells 7 days after delivery of the CRISPR-Cas9 RNP complexes containing sgRNA targeting B2M.
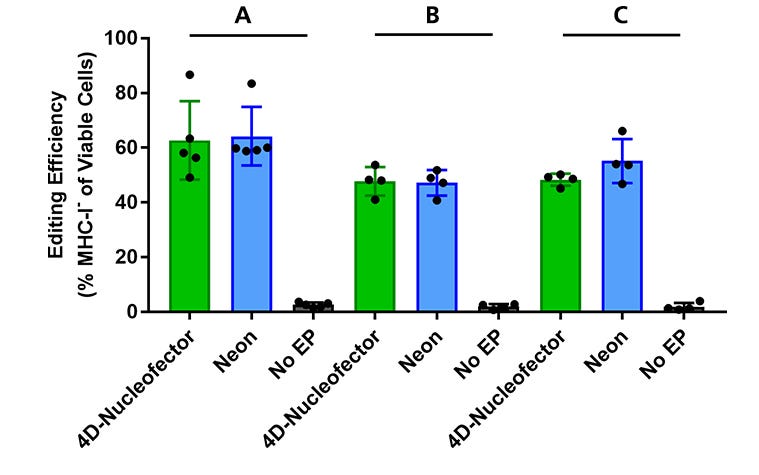
Figure 3. Validation of the Optimized RNP Delivery Electroporation Programs for Intestinal Organoid-Derived Cells Using the 4D-Nucleofector™ or Neon® Transfection Systems
Intestinal organoids were derived from healthy control biopsy samples (colon samples: lines A and B; small intestinal sample: line C) and cultured in IntestiCult™ Organoid Growth Medium (Human). RNP complexes containing ArciTect™ Cas9 Nuclease and ArciTect™ sgRNA targeting the B2M gene were delivered using either the 4D-Nucleofector™ System (program code: DS-138) or the Neon® Transfection System (1600 V, 20 ms, 2 pulses), and editing efficiency was monitored by flow cytometry to detect surface expression of major histocompatibility complex (MHC) class I molecules (MHC-I).
Note: Organoids from a variety of organs and disease models can be obtained from HUB Organoid biobank.- Document #PR00023
- Version 1.0.0
- July 2020
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration