In Vitro Drug Screening with the StemSpan™ Leukemic Cell Culture Kit
- Document # 27166
- Version 1.0.1
- May 2023
This Technical Bulletin describes the use of serum-free StemSpan™ medium and supplements to test the efficacy of novel drugs on hematopoietic stem and progenitor cells (HSPCs) isolated from patients with chronic myeloid leukemia (CML) or acute myeloid leukemia (AML).
Why Use StemSpan™ for Drug Screening?
- Culture conditions result in expansion and maintenance of CD34+ leukemic cells.
- Different readout methods, including flow cytometry or plate readers, can be used to assess changes to cell proliferation.
- Provides faster readout time (< 7 days) with simpler readout methods as compared to the cfu assay.
- Produces 50% inhibitory concentration (IC50) values similar to those determined by the CFU assay, the current standard in vitro assay for measuring hematotoxicity.
Background
Despite great advances in cancer diagnosis and treatment, leukemic stem and progenitor cells remain notoriously resistant to current therapies. As such, identification of novel treatments that are able to target leukemic stem cells (LSCs) is a major focus in leukemia research today.
The use of liquid culture assays to screen the efficacy of novel drugs on leukemic cells is often the first step prior to in vitro colony-forming unit (CFU) assays and in vivo studies. 1,2 Cultures of primary cells derived from CML and AML patient samples have been found recapitulate the complexity of the leukemic in vivo conditions more closely than cell lines, making them suitable for drug screening studies predictive of clinical outcomes. 3,4 However, obtaining primary cells in sufficient quantities for pre-clinical drug screening assays can be limited by the scarcity of patient samples.
The drug screening assay described in this protocol uses the StemSpan™ Leukemic Cell Culture Kit (Catalog #09720), which is able to expand and maintain CD34+ CML or AML cells. This liquid culture-based cytotoxicity assay allows drug testing in primary samples with low starting cell numbers as well as assessment of drug potency in eliminating LSCs. This protocol could also be extended to healthy bone marrow (BM) or umbilical cord blood (CB) comparing the toxicity and non-specificity of drug candidates on healthy and diseased cells. It may also be used to test the efficacy of a drug on specific patient samples for personalized treatment.
The 96-well plate format of this drug screening assay offers a quantitative, robust, and high-throughput readout. In addition, similar to the lineage-specific HemaTox™ (Catalog #09704) assay, results obtained from this liquid culture-based assay also correlate with the results obtained from CFU assays.3 Although in vitro CFU assays have traditionally been used for measuring drug potency and cytotoxicity, the liquid cultured-based assay has several advantages, including shorter turnaround time, ease of use, and flexibility. This allows researchers to narrow down their list of drug candidates quickly and with confidence before moving on to expensive and time-consuming assays, such as long-term culture-initiating cell (LTC-IC) assays or preclinical mouse models.
Media and Supplements for In Vitro Drug Screening Assay
The experiments presented in this Technical Bulletin were performed using the StemSpan™ Leukemic Cell Culture Kit, which contains StemSpan™ Serum-Free Expansion Medium II (SFEM II; Catalog #09605), StemSpan™ CD34+ Expansion Supplement (Catalog #02691) and the small molecule UM729 (Catalog #72332).
Other media and supplements, e.g. StemSpan™ SFEM (Catalog #09600), StemSpan™ CC100 (Catalog #02690) or CC110 (Catalog #02697), and small molecules, e.g. UM171, may also be used to generate similar culture conditions.
For more information on culturing CD34+ CML and AML cells using StemSpan™ media, refer to the Technical Bulletin: Culturing Leukemic Stem and Progenitor Cells with StemSpan™ Medium (Document#27105), available at www.stemcell.com.
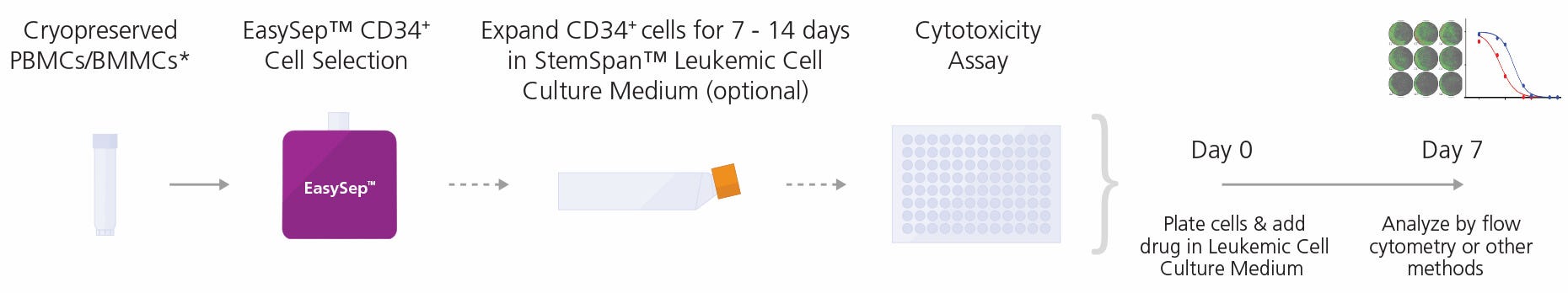
Figure 1. General Cytotoxicity Assay Procedure
CD34+ cells may be isolated from peripheral blood mononuclear cells (PBMCs) or bone marrow mononuclear cells (BMMCs) collected from patients with CML or AML using the EasySep™ Human Cord Blood CD34 Positive Selection Kit II (Catalog #17896)**. Cells can be further expanded for 7 - 14 days in StemSpan™ Leukemic Cell Culture Kit. Non-expanded or expanded cells are then cultured in StemSpan™ Leukemic Cell Culture Medium supplemented with control or test compounds in a 96-well plate at 37°C for 7 days prior to flow cytometry or other methods to determine IC50 values.
*The following experiments were performed on cryopreserved cells, however similar results are expected when using fresh samples.
**The EasySep™ Human Cord Blood CD34 Positive Selection Kit II (Catalog #17896) has been designed to work with fresh cord blood samples, but has been found to isolate CD34+ cells fresh or frozen PBMCs or BMMCs from CML or AML samples with suitable purity for this protocol.
Protocol
Method for In Vitro Drug Screening Assay
Both non-expanded and expanded CD34+ CML or AML cells can be used in drug screening to assess test compounds for their effects on the growth and proliferation of leukemic cells. Analysis methods in the drug screening assay may include automated cell counting, plate reader-based cell counting, or absolute cell counting of labeled cells (e.g. 7-AAD-, CD45+, or CD34+ cells) using a flow cytometer. For detailed protocols, refer to the product information sheet (PIS) for StemSpan™ Leukemic Cell Culture Kit (Document #10000003537), available at www.stemcell.com.
- Prepare StemSpan™ Leukemic Cell Culture Medium as described in the PIS (Document #10000003537).
- Dilute test compound in an appropriate solvent, to at least 1000X the concentration at which it will be tested in culture. This is the test compound stock solution.
- Prepare a 2X test compound solution by diluting the test compound stock solution (prepared in step 2) in StemSpan™ Leukemic Cell Culture Medium.
- To make a solvent control, dilute control solvent only in StemSpan™ Leukemic Cell Culture Medium to the same concentration as the solvent in the 2X test compound solution.
- Prepare non-expanded or expanded CD34+ CML or AML cells as described in the PIS.
- Immediately before plating, dilute cells in StemSpan™ Leukemic Cell Culture Medium to a concentration of 1000 - 8000 viable CD34+ CML/AML cells/100 μL (10,000 - 80,000 cells/mL).
- Add 100 μL of the cell suspension from step 6 to each well in a 96-well flat-bottom plate.
- Add 100 μL of the 2X test compound solution or solvent controls to wells. Pipette up and down gently to mix.
- Place each 96-well plate in a square dish (e.g. Catalog #38039) surrounded by 4 x 35 mm dishes (e.g. Catalog #27100) filled with sterile water.
- Incubate at 37°C in 5% CO2 and > 95% humidity for 7 days.
- Label cells with multiple surface markers (e.g. HSPC markers such as CD34 and/or CD45) for phenotyping by flow cytometry. Staining may be done in parallel with the ALDEFLUOR™ assay. Refer to PIS (Document #10000003537) for more details on labeling cells.
- Analyze by flow cytometry.
Analysis
Users can choose an appropriate readout method to measure the effect of test compounds on CD34+ CML or AML cell proliferation. The recommended method involves staining cultured cells for lineage-specific cell surface markers and counting cells expressing these markers by using a flow cytometer with absolute cell counting capability. This enables users to quantify the response and obtain estimates for the 50% and 90% inhibitory concentrations (IC 50 and IC90, respectively) of each test compound in the assay. Alternative readout methods (e.g. automated cell counting, imaging cytometry, or plate reader-based methods) can also be used, but may require further optimization.
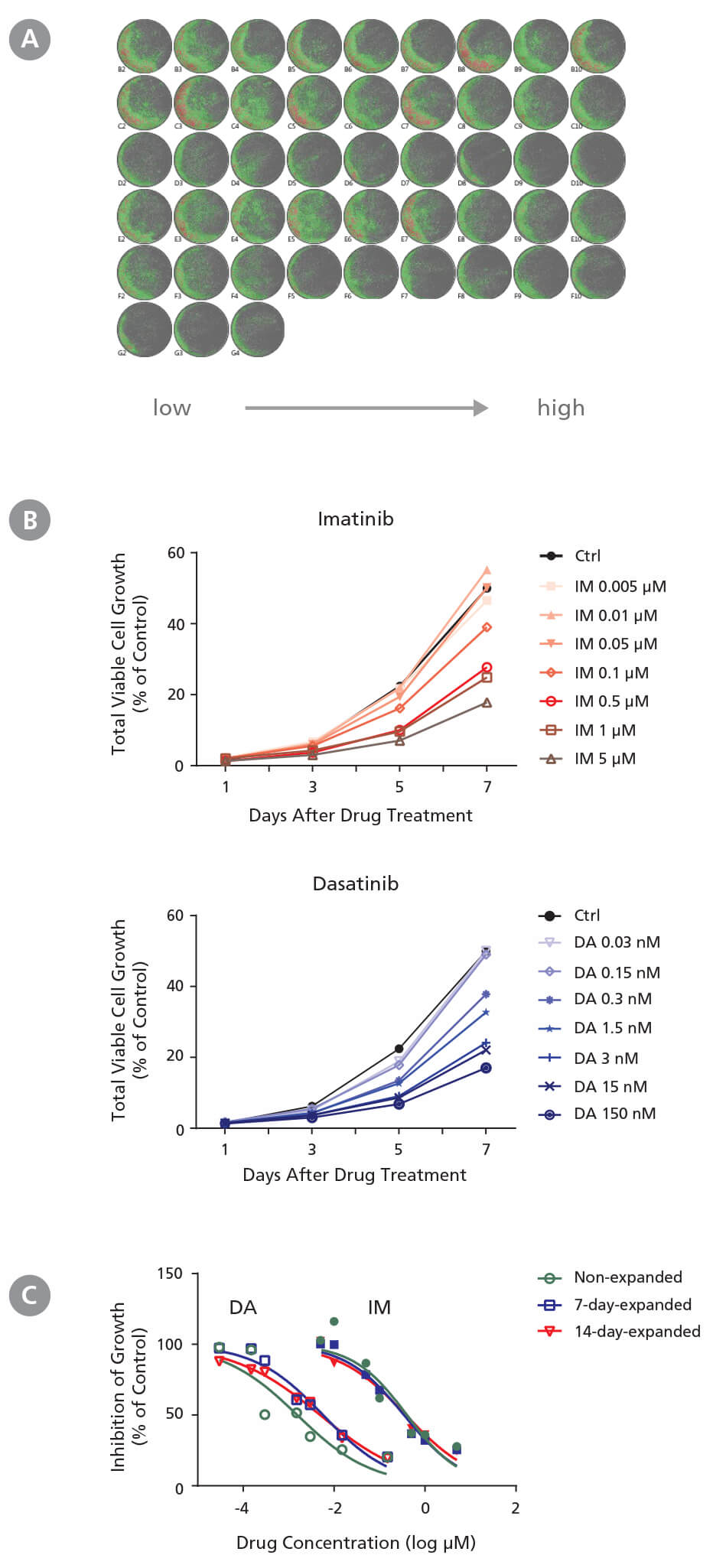
Figure 2. Drug Screening Assay for CML Cells Using StemSpan™ Leukemic Cell Culture Medium
CD34+ CML cells were treated with imatinib (IM) (Catalog #72532) and dasatinib (DA) (Catalog #73082) in StemSpan™ Leukemic Cell Culture Medium for 7 days. Cells were imaged every other day with a high-resolution imaging system to monitor cell growth, and were harvested at day 7 for flow cytometry analysis to measure IC 50. (A) Representative cell images (non-expanded cells) taken at day 7 post-IM and post-DA treatment from low to high concentrations in triplicates are shown. Cells are highlighted in green (low density) and red (high density). (B) Representative growth curves (non-expanded cells) with IM and DA treatments were generated from cell images taken every other day using the imaging system and its cell density algorithm. (C) Dose-response curves of non-expanded, 7-day expanded, and 14-day-expanded cells were generated using flow cytometry based on 7-AAD - cells at day 7 post drug treatment. Number of cells at each drug concentration were normalized to the number of cells in the solvent control (% of Ctrl). CD34 + CML cells respond to IM and DA in a dose- and time dependent manner. Both non-expanded and expanded CD34 + CML cells are useful for generating dose-response curves to determine IC 50 values. The average IC50 is 4.1 nM and 0.37 μM for IM and DA, respectively. Note both 7-day-expanded and 14-day-expanded CML cells still carry BCR-ABL genes and have similar BCR-ABL frequency as the non-expanded CML cells based on single colony qPCR (refer to Document #27105).
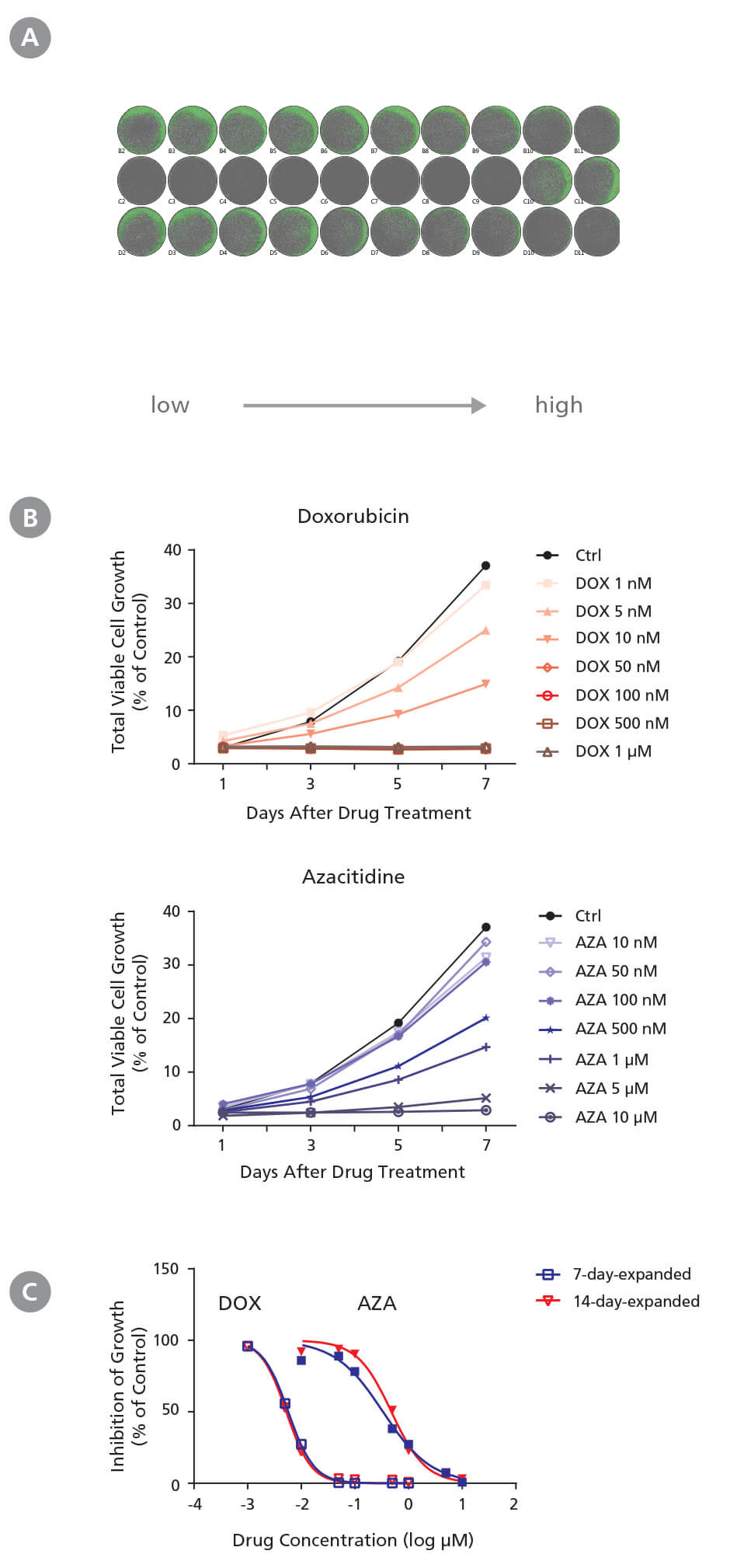
Figure 3. Drug Screening Assay for AML Cells Using StemSpan™ Leukemic Cell Culture Medium
CD34+ AML cells were treated with doxorubicin (DOX) and azacitidine (AZA) in StemSpan™ Leukemic Cell Culture Medium for 7 days. Cells were imaged every other day with a high-resolution imaging system to monitor cell growth, and were harvested at day 7 for flow cytometry analysis to measure IC 50. (A) Representative cell images (7-day-expanded cells) taken at day 7 post-DOX and post-AZA treatments from low to high concentrations in duplicates are shown. Cells are highlighted in green (low density) and red (high density). (B) Representative growth curves (7-day-expanded cells) with DOX and AZA treatments were generated from cell images taken every other day using the imaging system and its cell density algorithm. (C) Dose-response curves of 7-day-expanded and 14-day-expanded cells were generated using flow cytometry based on 7-AAD - cells at day 7 post drug treatment. Number of cells at each drug concentration were normalized to the number of cells in the solvent control (% of Ctrl). CD34 + AML cells respond to DOX and AZA in a dose- and time-dependent manner. Both non-expanded and expanded CD34 + AML cells are useful for generating dose-response curves to determine IC 50 values. The average IC50 is 5.5 nM and 0.41 μM for DOX and AZA, respectively.
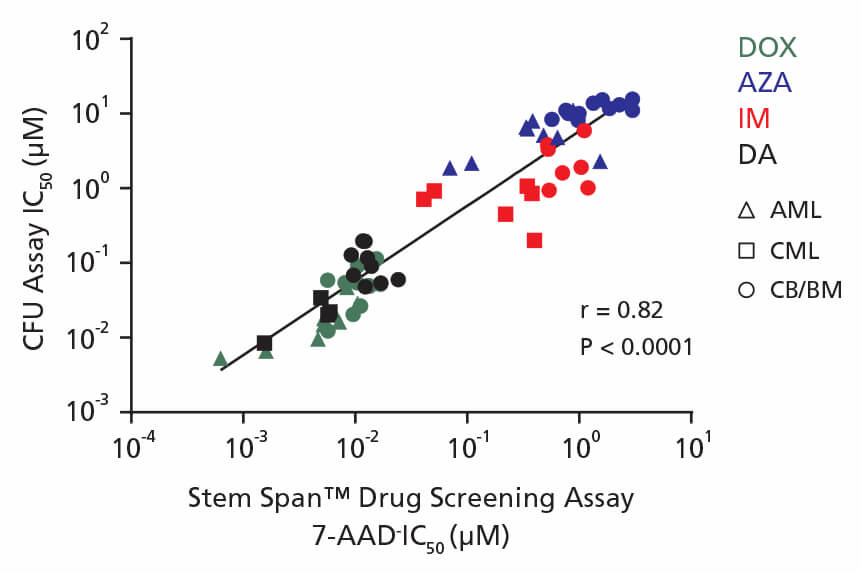
Figure 4. Correlation Between IC50 Values Using CFU Assay and the 96-Well Plate Liquid Culture-Based Cytotoxicity Assay
Non-expanded and expanded CML, AML, and CB CD34+ cells were cultured in colony-forming unit (CFU) assays using MethoCult™ H4435 Enriched medium and in liquid culture using StemSpan™ Leukemic Cell Culture Medium in the presence of four drugs: DOX (green), AZA (blue), IM (red), and DA (black). AML cells were treated with DOX and AZA; CML cells were treated with IM and DA. CB cells were treated with DOX, AZA, IM, and DA. Both non-expanded and expanded cells respond to drugs in a dose-dependent manner in the CFU assay and in drug screening assays. IC50 values generated using each assay were plotted on the x and y axes with a correlation coefficient (r) of 0.82 and P-value < 0.0001.
The experiments in this Technical Bulletin were performed using the small molecule UM171. This product is no longer licensed for sale by STEMCELL Technologies, however similar results are expected when using UM729 prepared to a final concentration of 1μM.For more information including data comparing UM171 and UM729, see Fares et al. 2014.
Products
For Isolation and Culture of CD34+ CML or AML Cells
*Similar results can be obtained using UM171 at a final concentration of 175 nM in culture.
For Analysis
*CD90 PE-Cy7 and CD45RA APC-Cy7 antibodies used in flow cytometry (Figure 4) were obtained from a different vendor.
References
- Lai D et al. (2018) PP2A inhibition sensitizes cancer stem cells to ABL tyrosine kinase inhibitors in BCR-ABL+ human leukemia. Science Translational Medicine 10(427).
- Minzel W et al. (2018) Small molecules co-targeting CKIα and the transcriptional kinases CDK7/9 control AML in preclinical models. Cell 175(1): 171-185.
- Uppal H et al. (2015) Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clinical Cancer Research 21(1): 123–133.
- Xing et al. (2012) Selective small molecule inhibitors of p110α and δ isoforms of phosphoinosityl-3-kinase are cytotoxic to human acute myeloid leukemia progenitors. Experimental Hematology 40(11): 922-933.
- Fares I et al. (2014) Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science 345(6203): 1509–1512.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration


