Generation of Erythroblasts from Pluripotent Stem Cells Using STEMdiff™ Media and Supplements
- Document # 27193
- Version 1.0.0
- Mar 2021
Background
Culture methods to differentiate human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells to erythroid cells are important for studying erythropoiesis during human development. The derivation of erythroid progenitor cells (erythroblasts) from human pluripotent stem cells (hPSCs) offers a potentially unlimited supply of erythroblasts and mature red blood cells (RBCs, also known as erythrocytes), for use in disease modeling, drug screening, and research into the regulation of erythropoiesis. In the future, erythrocytes derived from hPSCs may also become a safe alternative to donor RBCs for transfusion and cell-based therapies.
Existing methods for generating erythroblasts from hPSCs are technically challenging, involve multiple culture steps, have low cell yields, and lack reproducibility between cell lines and experiments. Here we describe a simple two-step, serum- and feeder-free culture system using STEMdiff™ Erythroid Kit to generate erythroblasts with high yields and purities in cultures of multiple hPSC lines.
Differentiate Human ES and iPS Cells to Erythroid Progenitor Cells
The STEMdiff™ Erythroid Kit is designed to differentiate hPSCs to erythroid progenitor cells, specifically transferrin receptor (CD71) and Glycophorin A (CD235, GlyA)-positive erythroblasts, in a 24-day, twostage protocol as shown in Figure 1. It comprises two culture media— STEMdiff™ Hematopoietic Basal Medium and StemSpan™ SFEM II—and the following three supplements:
- STEMdiff™ Hematopoietic Supplement A: Promotes mesodermal specification of hPSCs
- STEMdiff™ Erythroid Supplement E1: Promotes specification of hPSCs to hematoendothelial and hematopoietic lineages
- STEMdiff™ Erythroid Supplement E2: Promotes expansion and differentiation of hematopoietic progenitor cells to erythroblasts
Why Use STEMdiff™ for Generating Erythroblasts?
- Eliminate variation introduced by serum and feeder-cells by using serum- and feeder-free conditions.
- Produce thousands of erythroblasts per input human ES/iPS cell.
- Further mature ES/iPS cell-derived erythoblasts using our optional protocol.
- Achieve robust generation of erythroblasts across multiple ES and iPS cell lines.
Protocol for Generating Erythroblasts
The following protocol is recommended for the expansion and erythroid differentiation of hPSCs maintained in mTeSR™1, mTeSR™ Plus, or TeSR™-E8™.
Using this protocol, cell yields may reach 100,000 erythroblasts per input hPSC in 24 days; however, this number may vary between experiments depending on the cell line and condition of the cells at the start of the differentiation culture. Depending on your specific objectives, further protocol optimization (e.g. testing of different plating densities, culture periods, and feeding and replating schedules) may be required.
With this protocol, typically more than 70% of cells generated after 24 days of culture are CD71+GlyA+ erythroblasts. Immature CD71+ GlyA-erythroid progenitor cells and pro-erythroblasts, and more-differentiated CD71low/GlyA+ normoblasts may also be present. Further erythroblast maturation may be achieved by replating cells in medium containing erythropoietin (EPO; Catalog #78007) as a single growth factor. Refer to the optional erythroid maturation protocol for further details.
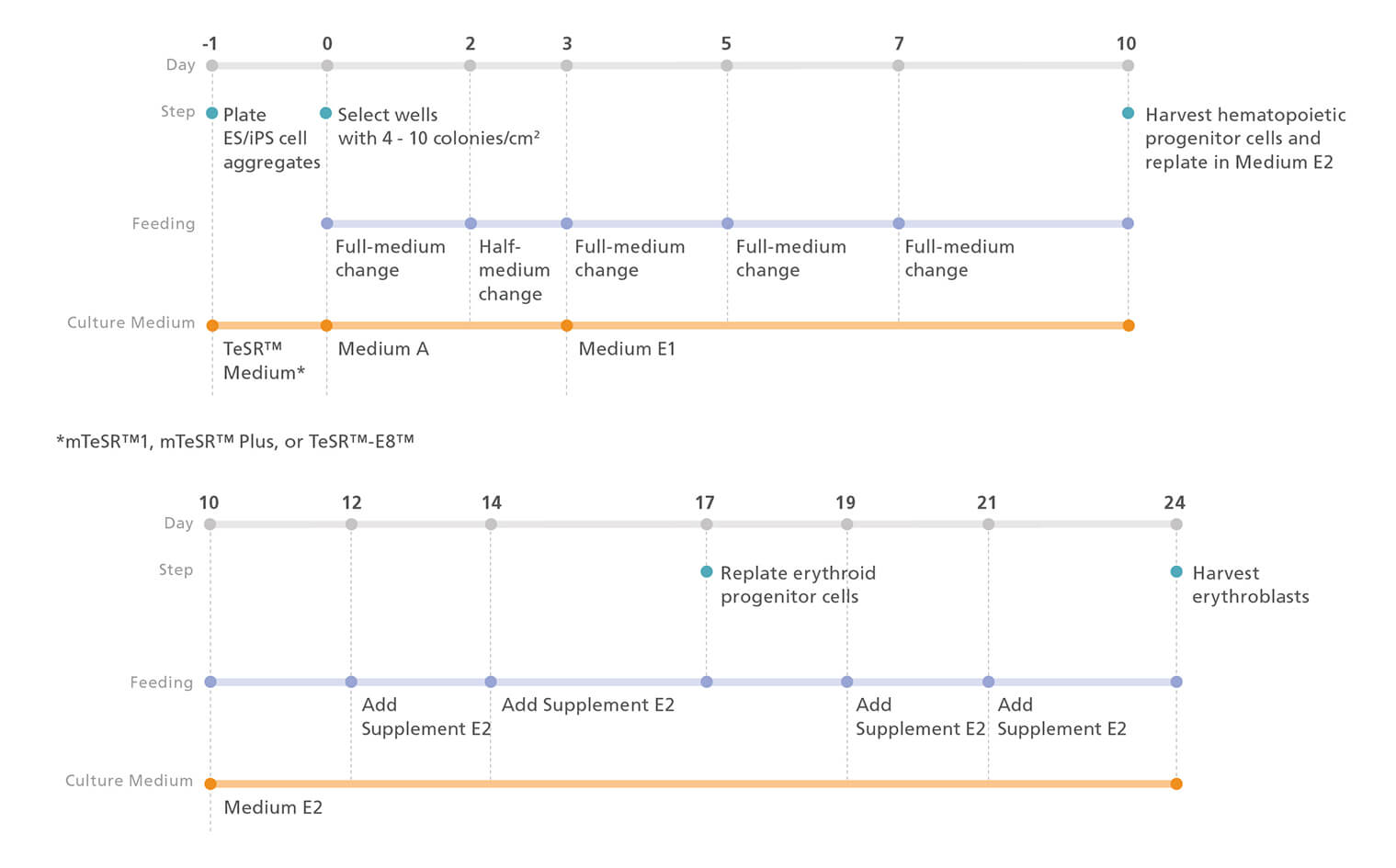
Figure 1. Erythroid Differentiation Protocol
The protocol involves two main steps: (A) Hematopoietic specification of hPSCs and (B) differentiation of hPSC-derived hematopoietic progenitor cells to erythroblasts. The diagrams show key timepoints; for further details, see the protocol below.
Protocol for Differentiation of hPSCs to Erythroblasts
This procedure has been optimized for use with human ES and iPS cells; refer to the Product Information Sheet (PIS; Document #10000007684) for complete instructions.
- Passage hPSC aggregates and set up for differentiation as per
the protocol in the PIS.
Note: For complete instructions on maintaining high-quality human ES and iPS cells and for coating plates with Corning® Matrigel®, refer to the Technical Manual for mTeSR™1, mTeSR™ Plus, or TeSR™ E8™, available at www.stemcell.com, or contact us to request a copy.
Hematopoietic Differentiation Protocol
Note: Throughout the protocol, warm all media to room temperature (15 - 25°C) before use. Do not leave the media at room temperature for extended periods of time.- On Day 0, confirm that 16 - 40 colonies/well are adhered to the cultureware (4 - 10 colonies/cm2 ). This protocol is based on 12-well plates, but can be adapted for other cultureware sizes. Aspirate medium from wells.
- Add 1 mL of Medium A (STEMdiff™ Hematopoietic Basal Medium + STEMdiff™ Hematopoietic Supplement A) per well. Store remaining Medium A at 2 - 8°C until required.
- Incubate at 37°C for 2 days.
- On Day 2, gently remove 0.5 mL of medium from each well and discard. Gently add 0.5 mL of Medium A per well.
- Incubate at 37°C for 24 hours.
- On Day 3, aspirate medium from wells. Gently add 1 mL of Medium E1 (STEMdiff™ Hematopoietic Basal Medium + STEMdiff™ Erythroid Supplement E1) per well. Store remaining Medium E1 at 2 - 8°C until required.
- Incubate at 37°C for 2 days.
- Perform a full-medium change with Medium E1 on Days 5 and 7 as per the PIS.
Note: Cells may be harvested on Day 12 instead of Day 10. However, the optimal harvest day may be cell line-dependent. - Harvest hematopoietic cells: If harvesting on Day 12, proceed
as follows on Day 10; if harvesting on Day 10, skip this step
and proceed to step 10.
- a. Keep the plate flat and, using a serological pipette or a 1 mL pipette tip, gently remove 0.5 mL of medium from each well and discard.
- b. Add 0.5 mL of Medium E1 per well.
- c. Incubate at 37°C for 2 days.
- Harvest suspension cells as follows:
- a. Pipette the cells up and down to wash the well and remove the suspension cells from the adherent cell layer.
- b. Add 1 mL of StemSpan™ SFEM II to the well. Triturate vigorously in the well and add to the collection tube.
- c. Repeat steps 10a and 10b for each well.
- d. Centrifuge collection tubes at 300 x g for 5 minutes at room temperature (15 - 25°C).
- e. Remove and discard the supernatant.
- f. Resuspend the cell pellet in StemSpan™ SFEM II and perform a viable cell count using Trypan Blue and a hemocytometer.
Follow this protocol for step-by-step instructions on performing viable cell counts with a hemocytometer and Trypan Blue.
Erythroid Differentiation Protocol
- On Day 10, add 1 mL of Medium E2 (StemSpan™ SFEM II + STEMdiff™ Erythroid Supplement E2) to each well of a tissue culture-treated 12-well plate.
- Add harvested cells (from step 10 of the Hematopoietic Differentiation Protocol) to each well at 40,000 cells/mL. Incubate at 37°C.
- After 2 days (Day 12), add 100 µL of Supplement E2 per well. Incubate at 37°C.
- Following 2 more days (Day 14) of incubation, add 100 µL of Supplement E2 per well. Incubate at 37°C for 3 days.
- Passage cells as follows on Day 17:
- a. Using a serological pipette or a 1 mL pipette tip, gently pipette the cells up and down to wash the well.
- b. Transfer the cell suspension to a collection tube and centrifuge at 300 x g for 5 minutes at room temperature.
- c. Discard the supernatant.
- d. Resuspend the cell pellet in StemSpan™ SFEM II and perform a viable cell count using Trypan Blue and a hemocytometer.
- e. Add 1 mL of Medium E2 per well of a 12-well plate.
- f. Add cells to each well at 167,000 cells/mL. Incubate at 37°C for 2 days.
- Repeat steps 3 and 4 on Days 19 and 21.
- Harvest cells on Day 24 as follows:
- a. Using a serological pipette or a 1 mL pipette tip, gently pipette the cells up and down to wash the well.
- b. Transfer the cell suspension to a collection tube.
- c. Centrifuge the tube at 300 x g for 5 minutes at room temperature. Remove and discard the supernatant.
- d. Resuspend the cell pellet in desired medium and perform a viable cell count for analysis or downstream assays. If desired, the purity of the CD71+GlyA+ erythroid cell population can be determined by flow cytometry.
- Assess erythroid differentiation; the following antibodies are
recommended for assessment of hPSC-derived erythroblasts
by flow cytometry:
- Anti-Human CD71 (Transferrin Receptor) Antibody, Clone OKT9 (Catalog #60106)
- Anti-Human CD235a (Glycophorin A) Antibody, Clone 2B7 (Catalog #60152)
Erythroid Maturation Protocol (Optional)
Cells generated using the STEMdiff™ Erythroid Kit have the capacity to mature into normoblasts and reticulocytes when they are moved to appropriate culture conditions for maturation. The following is a suggested maturation protocol:
- Prepare maturation medium by mixing the following growth factors with StemSpan™ SFEM II:
- 3 U/mL Human Recombinant EPO (Catalog #78007)
- 3% human AB serum
- Wash hPSC-derived erythroblasts with either Iscove’s MDM or StemSpan™ SFEM II.
- Centrifuge at 200 x g for 5 - 10 minutes. Remove and discard the supernatant.
- Resuspend cells at a concentration of 5 - 10 x 105 cells/mL in fresh maturation medium. Add 1 mL per well of a 12-well plate and incubate at 37°C for 3 - 4 days.
- Add 1 mL of fresh maturation medium per well. Incubate at 37°C for 3 - 4 days.
- After 7 days, harvest cells as described in step 7 of the Erythroid Differentiation Protocol.
Results
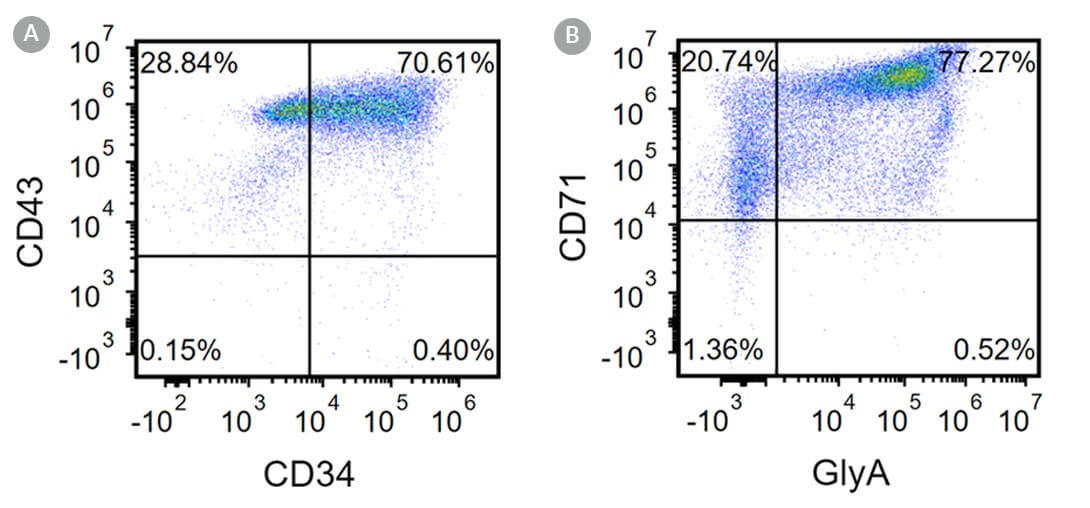
Figure 2. Robust and Efficient Generation of CD71+GlyA+ Erythroblasts Using the STEMdiff™ Erythroid Kit
Human ES and iPS cells were induced to differentiate to CD71+GlyA+ cells using the 24-day protocol shown in Figure 1. Following the initial 10-day culture period and at the end of the 24-day protocol, cells were harvested from the supernatant and analyzed by flow cytometry. Dead cells were excluded by lightscatter profile and propidium iodide staining. (A) Representative flow cytometry plot for iPS cell-derived (WLS-1C) cells analyzed on Day 10. Cells were identified as CD34+ CD43+ hematopoietic progenitors. (B) After 24 days of culture, cells were identified as having differentiated to CD71+GlyA+ erythroblasts. Erythroid identity of the generated cells was additionally assessed by cell morphology and hemoglobin expression (see Figure 4).
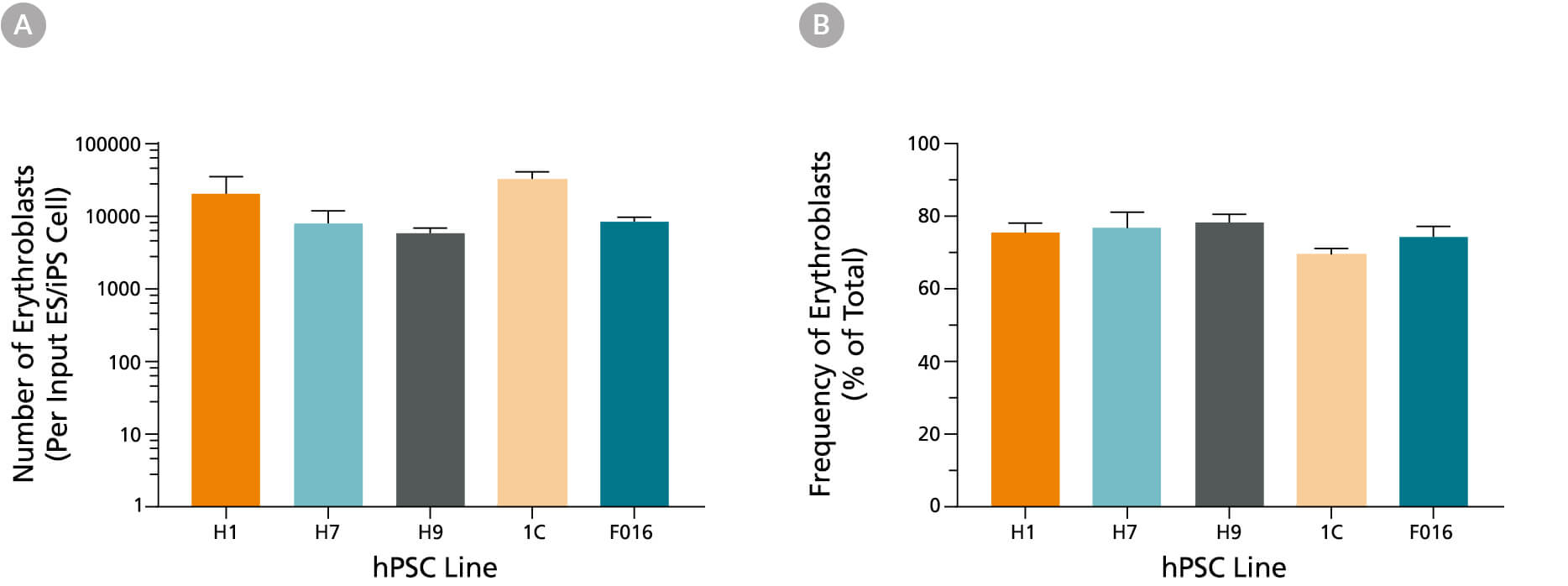
Figure 3. Production of Erythroblasts from Human iPS and ES Cells After 24 Days of Culture Using the STEMdiff™ Erythroid Kit
(A) Average numbers of erythroblasts generated after culturing human ES and iPS cells for 24 days using the STEMdiff™ Erythroid Kit. Shown are the numbers of erythroblasts that express GlyA per input hPSC. (B) The overall frequency of GlyA+ erythroblasts present in culture after 24 days is shown as a percentage of total live cells present. Data are presented as mean and SEM (n = 5 - 21) for six individual hPSC lines.
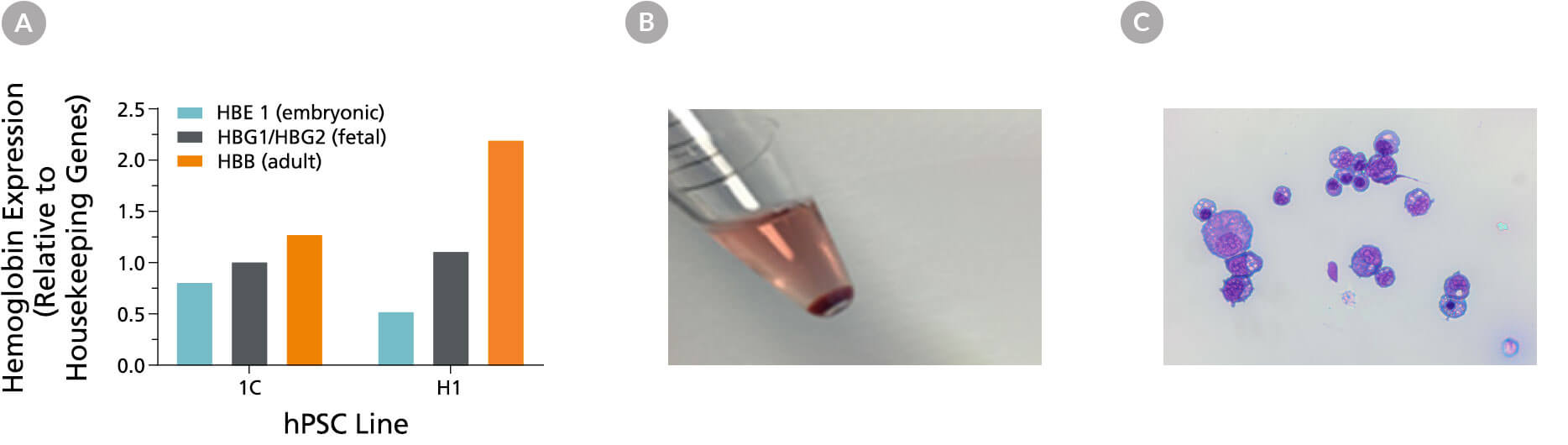
Figure 4. hPSC-Derived Erythroblasts Are Hemoglobinized and Display Typical Erythroid Morphology
(A) Erythroblasts generated from hPSC-derived hematopoietic progenitor cells express a mix of primitive (embryonic) and definitive (fetal and adult) hemoglobin. The results of qPCR analysis for globin gene expression are shown after 24 days of culture. (B) An image of the red cell pellet shows that cells produced in culture are hemoglobinized. (C) Cells display typical basophilic erythroblast morphology after 24 days of culture using the STEMdiff™ Erythroid Kit (40X magnification, May-Grunwald Giemsa stain).
Optional Erythroid Maturation
Erythroblasts derived from hPSCs can be further matured during 7 days of culture under maturation conditions in StemSpan™ SFEM II supplemented with 3 U/mL EPO + 3% human AB serum. At the end of the culture period, the cells were harvested, counted, and assessed for expression of erythroid markers CD71 and GlyA, and changes in cell morphology.
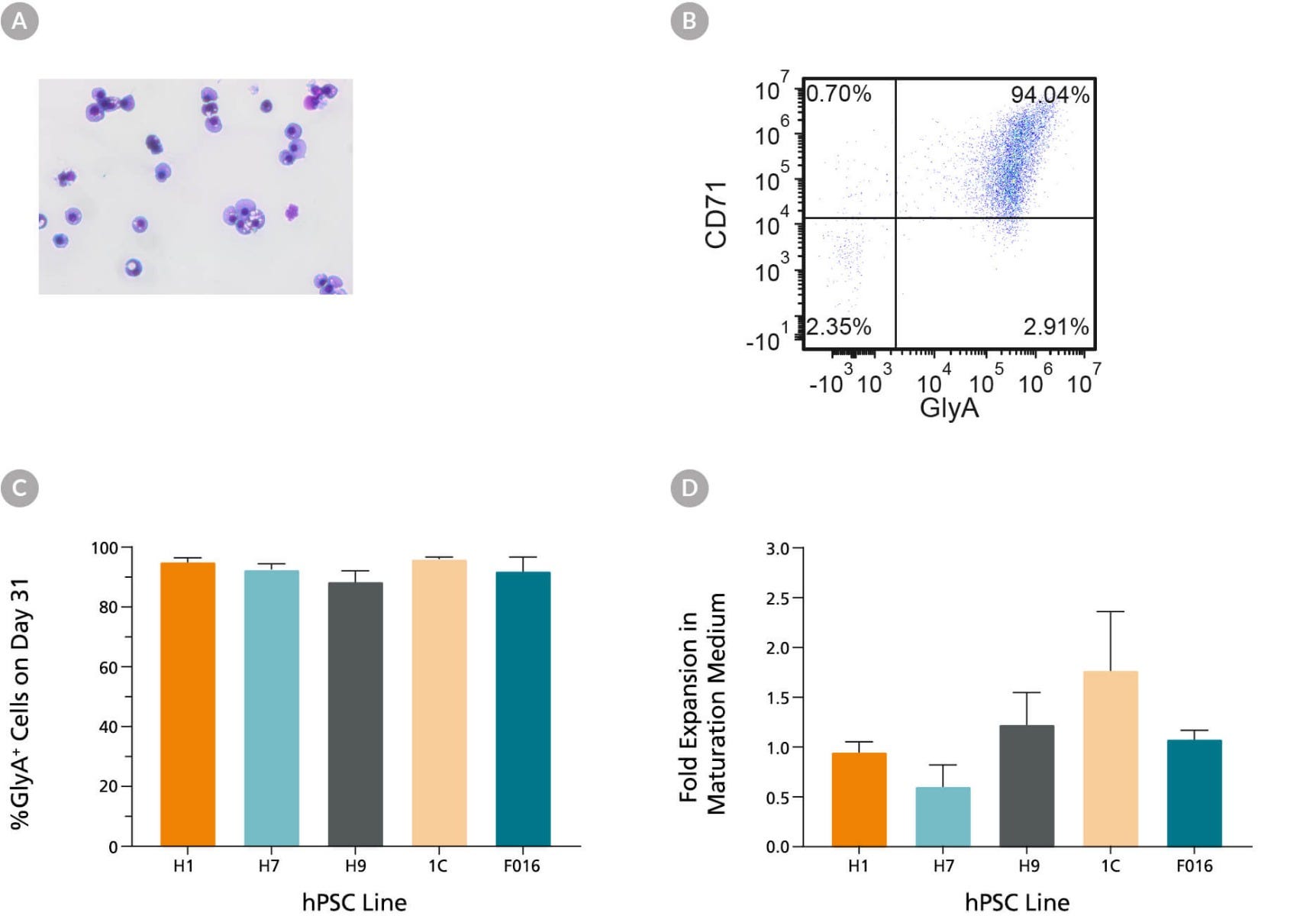
Figure 5. hPSC-Derived Erythroblasts Can Mature Into CD71low/GlyA+ Normoblasts
hPSC-derived erythroblasts can further mature when cultured for an additional 7 days in StemSpan™ SFEM II supplemented with human AB serum (3%) and EPO (3 U/mL). (A) Further maturation of erythroblasts results in smaller cells with condensed nuclei, which is typical for orthochromatic normoblasts (40X magnification, May-Grunwald Giemsa stain). (B) Representative flow cytometry plot shows that over 90% of cells are GlyA+ and the majority of cells have decreased CD71 expression as compared to erythoblasts (see Fig 2b), which is consistent with erythroid maturation. (C) Bar graphs summarize the average frequencies of GlyA+ erythroblasts on Day 31 across five hPSC lines. (D) Notably, fold expansion data show that cell numbers are maintained during the maturation culture. Data are presented as mean and SEM (n = 2 - 6).
Product Information
Materials Required But Not Included
Recommended Antibodies for Analysis
Materials Recommended for Further Maturation
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration

