How to Count Cells with a Hemocytometer
How to perform total nucleated cell counts with 3% Acetic Acid with Methylene Blue, and how to perform viable cell counts by Trypan Blue dye exclusion
Cell counting can be performed using Trypan Blue or 3% Acetic Acid with Methylene Blue. When performing a total nucleated cell count, 3% Acetic Acid with Methylene Blue is recommended. Acetic acid lyses the cellular membranes, and the methylene blue stains the exposed nuclei. Because mature red blood cells lack nuclei, they are excluded when counting. Alternatively, Trypan Blue is recommended for counting viable mammalian cells. Trypan Blue penetrates the cell membrane, thus it enters the cytoplasm of cells with compromised membranes (dead cells) to stain them blue. The live cells remain intact and can be distinguished from dead cells by their ability to exclude the blue dye. In this case one would count the intact viable cells.
This protocol describes how to perform total nucleated cell counts with 3% Acetic Acid with Methylene Blue, and how to perform viable cell counts by Trypan Blue dye exclusion. Additonal cell counting resources and templates to help streamline your assays are also available.
Materials
- 3% Acetic Acid with Methylene Blue (Catalog #07060) or Trypan Blue (Catalog #07050)
- 96-well plate (e.g. Corning® 96-Well Round-Bottom Microplate, Catalog #38018) or microcentrifuge tube (e.g. Costar® Microcentrifuge Tubes, Catalog #38090)
- Hemocytometer and coverslip (e.g. Hausser Scientific™ Bright-Line Hemocytometer, Catalog #100-1181)
- 70% Ethanol
- Pipettor (e.g. Corning® Lambda™ Plus Pipettor, Catalog #38060)
Protocol
Part I: Sample Preparation
-
Prepare an appropriate dilution of the well-mixed single-cell suspension using phosphate-buffered saline or serum-free medium. For an accurate representation of concentration, use at least 20 µL of cell suspension to make the dilution.
Example: Preparing a 10-fold dilution
a.Add 180 µL of 3% Acetic Acid with Methylene Blue to a microcentrifuge tube or well of a 96-well plate.
b.Mix the cell suspension and add 20 µL to the tube or well containing 3% Acetic Acid with Methylene Blue.
- Ensure that the cell suspension to be counted is completely resuspended. Before the cells settle, place a suitable volume of a cell suspension (20 - 200 μL) in a centrifuge tube.
- Add an equal volume of 0.4% Trypan Blue and gently mix.
- Incubate the mixture at room temperature (15°C – 25°C) for 5 minutes.
Part II: Cell Counting with a Hemocytometer
- Prepare a hemocytometer by cleaning the chamber surface with 70% ethanol. Wipe dry. Position the coverslip over the chambers.
-
Resuspend the cell mixture and place 10 μL of stained cells into the hemocytometer chamber using a 20 µL pipettor.
Note: Be careful not to move the coverslip. Allow capillary action to draw the sample in. - Place the hemocytometer on the stage of a binocular light microscope. Adjust the microscope to 10X magnification and focus on the cells.
-
Using a hand tally counter, count the cells (stained nuclei) in each of the four outside squares of the hemocytometer (Figure 1A), including cells that lie on the bottom and left-hand perimeters, but not those that lie on the top and right-hand perimeters (Figure 1B). If 3% Acetic Acid with Methylene Blue was used in Part I, count the stained cell nuclei. If Trypan Blue was used in Part I, count the unstained viable cells.
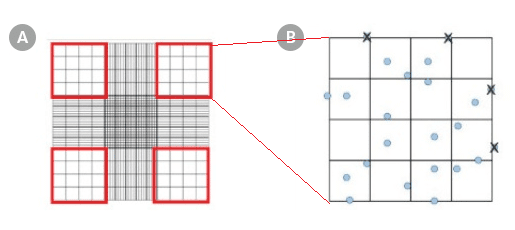
Figure 1. Hemocytometer Gridlines
Hemocytometer diagram indicating the (A) four sets in red and (B) the 16 squares within one of the sets that should be used for counting.
Note: The appropriate dilution factor will depend on the approximate number of cells present in the starting sample but should result in a cell concentration that gives 50 - 100 cells per square (i.e. large or major square) in the hemocytometer. If there are more or fewer than approximately 100 cells per major square on the hemocytometer, prepare a new diluted sample using a greater or smaller dilution factor. -
Each of the nine major squares of the hemocytometer represents a total volume of 0.1 mm3. Since 1 cm3 is equivalent to 1 mL, the cell concentration can be determined using the following equation:
Total number of nucleated cells/mL = average cell count per square x dilution factor x 104Example: If the cell counts for each of the four outer squares were 21, 15, 20, and 17 at a 100 dilution factor then the average cell count would be (21 + 15 + 20 + 17) ÷ 4 = 18.25.
Therefore, the total concentration of cells in the original suspension would be: 18.25 x 100 x 10^4 = 18,250,000 cells/mL
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration