Isolating Extracellular Vesicles from Urine with EasySep™ EV Human Positive Selection Kits
- Document # 27249
- Version 1.0.0
- Jan 2024
Extracellular vesicles (EVs), including apoptotic bodies, microvesicles, and exosomes, are structures naturally released from cells that are delimited by a lipid bilayer. EVs differ not only in size, but also in composition and subcellular origin. Additionally, they function in intercellular communication under homeostatic and pathological conditions and are frequently characterized by the expression of the tetraspanin proteins CD9, CD63, and CD81.1 EVs contain protein, nucleic acid, enzyme, cytokine, or lipid cargoes that reflect the state of the cell of origin. EV cargo serves a broad range of biological functions, including protein quality control, cell polarity, remodeling of the extracellular matrix (ECM), intercellular signaling, and transfer of biological materials. Importantly, EV cargo may impact the phenotype and function of the recipient cell. Thus, along with the study of fundamental EV biology, there is growing interest in studying the composition of EV cargoes to identify diagnostic or prognostic biomarkers of disease, as well as to assess the therapeutic potential of EVs. This technical bulletin describes a protocol to efficiently isolate urine-derived EVs based on tetraspanin expression, achieving both high EV yield and purity, using the EasySep™ EV Human Positive Selection Kits (CD9, CD63, CD81, and Pan Kits).
Urine-Derived EVs: a Biomarker Source for Developing Diagnostic and Prognostic Tools
Urine is a body waste fluid that can be easily obtained and is thus an ideal source for biomarker analysis. As a dynamic, bioactive fluid, urine can provide information about an individual's health status. As urine is already a valuable diagnostic medium, there is growing interest in urinary EV analysis. Specifically, EVs found in urine reflect the state of the urinary system, from podocytes to renal tubular cells, making them an excellent sample source to study kidney physiology and pathology. Urinary EVs are secreted from all cell types facing the urinary space (e.g. glomerulus, renal tubule, and the cells lining the urinary tract). As a result, urinary EVs can provide a non-invasive image of the physiological state of the renal tubular system.2,3 Alterations in urinary EV composition are of special interest since they may provide information about disease pathophysiology and diagnostic end points to study renal disease. As such, urine is considered the perfect source of biomarkers for developing new diagnostic tools to identify and stratify patients suffering from kidney failure and/or renal diseases.4
EV Isolation Methods: EasySep™ vs. Differential Ultracentrifugation
In order to study EVs, most studies found in the literature use differential ultracentrifugation (UC) to perform EV isolation. UC utilizes size to separate EVs from other contaminants such as cellular debris and contaminating proteins. UC protocols for isolating small EVs usually involve three centrifugation steps. First, cells are removed through low-speed centrifugation at 2000 x g. Next, samples are pre-cleared from both cellular debris and large EVs using intermediate-speed centrifugation at 10,000 - 17,000 x g. Then, a pellet of small EVs is obtained through high-speed centrifugation at 100,000 - 200,000 x g. Unfortunately, UC is a laborious and time-consuming method that requires expensive ultracentrifugation equipment. This technical bulletin describes a protocol for EV isolation from urine using EasySep™, a more efficient and easy-to-use alternative to UC. EasySep™ technology targets EVs using tetrameric antibody complexes that recognize tetraspanin proteins. The antibody complexes link EVs to EasySep™ magnetic particles, and labeled EVs are then separated by placing the tube containing the sample into an EasySep™ magnet. The labeled EVs remain in the tube while unwanted biofluid components are poured off.
Why Use EasySep™ EV Human Positive Selection Kit to Isolate EVs from Urine?
- Avoid ultracentrifugation and associated time-consuming EV isolation methods
- Obtain higher-purity samples compared to ultracentrifugation-based EV isolation methods
- Achieve greater EV yield compared to ultracentrifugation-based EV isolation methods
- Bypass expensive ultracentrifugation equipment
- Choose your sample volume
Testing EV Isolation Performance: EasySep™ vs. Differential Ultracentrifugation
To compare EasySep™- and UC-based EV isolation efficiency, both EV population recovery and purity were assessed using western blot (Figure 1). EV isolation from urine samples was performed either with the EasySep™ Human Pan-Extracellular Vesicle Positive Selection Kit (Catalog #17891), which targets all three tetraspanin markers (CD9, CD63, and CD81) and follows the Protocol for EasySep™ EV Isolation from Urine described in this bulletin, or through two rounds of differential UC at 100,000 x g for 70 minutes. Western blot analysis demonstrated that EasySep™ EV isolation outperforms the differential UC method as it enhances both EV recovery and EV purity (Figure 1).
Once EV isolation efficiency was confirmed using EasySep™ Pan-Extracellular Vesicle Positive Selection Kit (Catalog #17891), a second experiment was performed to assess its effectiveness using the single tetraspanin EV positive isolation kits (i.e. EasySep™ Human Extracellular Vesicle Positive Selection Kits for CD81, CD63, and CD9; Catalogs #17892, #17895, and #17894, respectively). As expected, western blot revealed that single tetraspanin kits preferentially enrich the EVs expressing the corresponding tetraspanin from each kit (i.e. the CD9 kit enriched the CD9+ EV population, whereas CD63 and CD81 kits enriched the CD63+ and CD81+ EV populations, respectively; Figure 2). Importantly, EVs do not always express all three tetraspanins. Therefore, it is likely that the single tetraspanin kits only isolated certain EV subpopulations from the total EV population present in the sample (Figure 2).
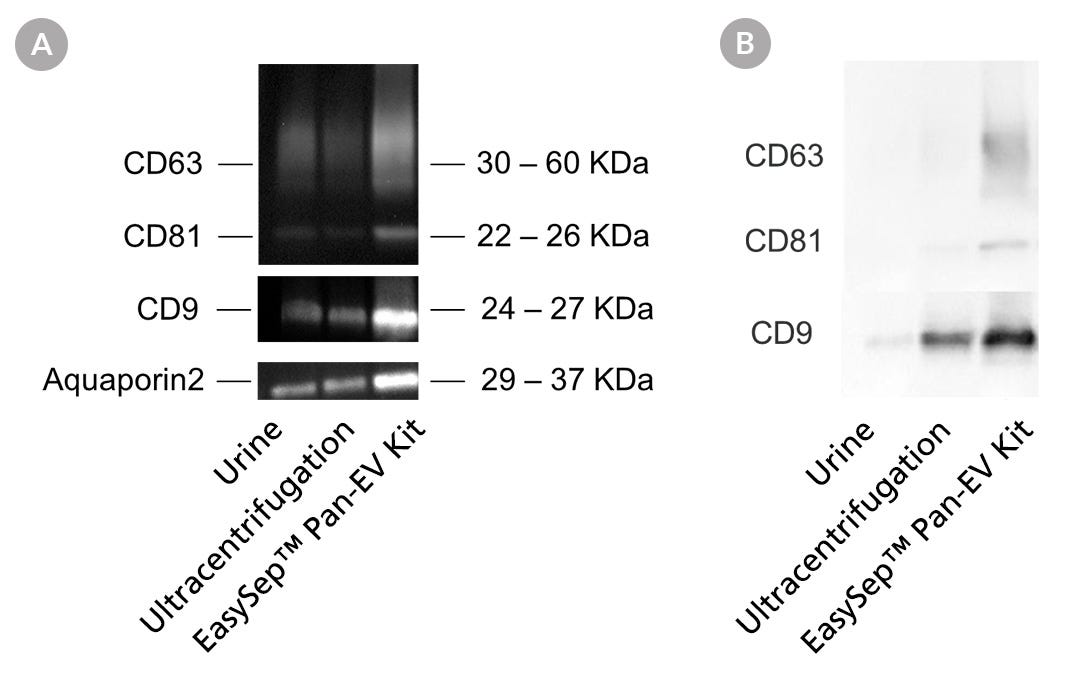
Figure 1. EasySep™ Achieves Higher EV Recovery and Purity from Urine Samples Compared to the Differential Ultracentrifugation Method
EVs were isolated from urine using either UC or the EasySep™ Human Pan-Extracellular Vesicle Positive Selection Kit (Catalog #17891) following the Protocol for EasySep™ EV Isolation from Urine described in this technical bulletin. Isolated EVs were analyzed by western blot. A. EV recovery comparison. From left to right, the first lane was loaded with 35 µL of normal urine (i.e. unenriched/unconcentrated control). The next two lanes depict samples of EVs isolated using UC and EasySep™, respectively, from 8 mL of input urine volume. Following EV isolation, 250 µL of PBS was added to resuspend each pre-concentrated sample and normalize them all to equal volumes. Next, 35 µL of sample was loaded per lane. Membranes were then probed for CD9, CD63, CD81, and Aquaporin 2 (kidney-specific marker 5, 6) antibodies. Results show that the EasySep™ EV Human Positive Selection Kit produces higher EV yield compared to UC. B. EV purity comparison. The same set of samples from Figure 1A was first analyzed by bicinchoninic acid (BCA) assay to determine the total protein concentration of samples. Then, the same amount of protein (0.5 μg) from each sample was analyzed by western blot to compare the relative proportion of EV proteins to total proteins. Higher tetraspanin intensity per microgram of total protein equates to higher EV purity. Samples were loaded in the same order as described in Figure 1A. Membranes were then probed for CD9, CD63, and CD81. Results show that the EasySep™ EV Human Positive Selection Kit produces purer EVs compared to UC.
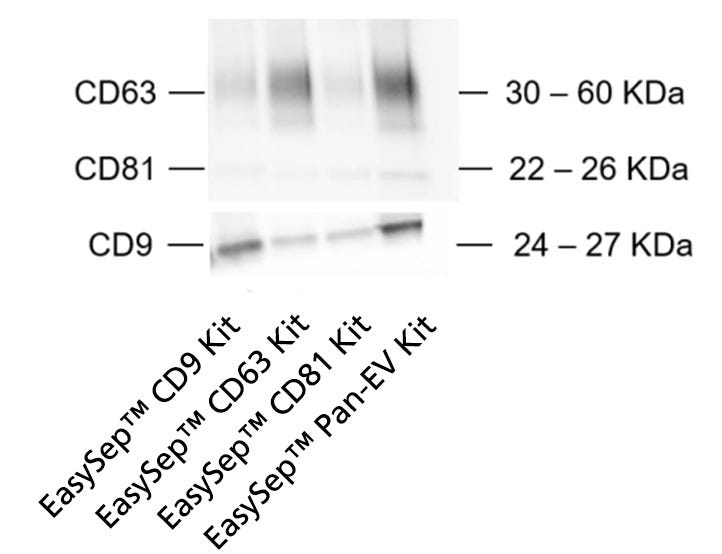
Figure 2. The Protocol for EasySep™ EV Isolation from Urine May Be Used to Isolate Individual EV Subpopulations Using the EasySep™ Single Tetraspanin Kits
To investigate the use of the Protocol for EasySep™ EV Isolation from Urine for isolation of EV subtypes, EV isolation was tested with three additional EasySep™ kits targeting single tetraspanin markers (CD81, CD9, or CD63). EV subtypes isolated with the EasySep™ Human Extracellular Vesicle (CD81), EasySep™ Human Extracellular Vesicle (CD63), and EasySep™ Human Extracellular Vesicle (CD9) Positive Selection Kits were compared to EVs isolated with the EasySep™ Human Pan-Extracellular Vesicle Positive Selection Kit. Each sample was obtained from 8 mL of input urine volume. Following EV isolation, 250 µL of PBS was added to resuspend each pre-concentrated sample and normalize them all to equal volumes. Next, 35 µL of sample was loaded per lane and analyzed by western blot to compare CD9, CD63, and CD81 expression. Results reveal that single tetraspanin kits preferentially enrich the EVs expressing the targeted tetraspanin from each kit, thus confirming that the Protocol for EasySep™ EV Isolation from Urine may be used to isolate EV subtypes.
Protocols for Urine Sample Processing and EV Isolation
To first process the samples, freshly collected urine was briefly centrifuged to remove cells before further sample processing and EV isolation. If urine samples are not immediately used, it is recommended to freeze them for long-term storage7. The Protocol for EasySep™ EV Isolation from Urine can be used to isolate EVs from both fresh and frozen urine (Figure 3).
To finish processing the samples, the Sample Processing Protocol below includes an optional pre-clearing step for researchers who would like to remove large EVs and/or improve EV purity. Pre-clearing helps remove Tamm-Horsfall urinary glycoprotein (THP) aggregates, also known as uromodulin, an abundant protein in urine and a common contaminant in urinary EV isolations. However, pre-clearing may also remove large EVs that may express CD9, CD63, and CD81, which results in slightly lower EV yields. (Figure 4).
Urine for EV isolation can be collected in a wide variety of volumes ranging from 1 - 30 mL depending on the collection methods.7 The Protocol for EasySep™ EV Isolation from Urine may be used to isolate EVs from highly concentrated samples of up to 20 mL of urine pre-concentrated to 1 mL before EasySep™ isolation. In order to process large urine volumes before EV isolation, the Protocol for EasySep™ EV Isolation from Urine recommends a concentration step using a 100K centrifugal filter tube (e.g. PALL® Macrosep® Advance Centrifugal Devices with Omega Membrane [PALL® filter]).
Did You Know?
- Pre-concentrating urine samples with volumes larger than 2 mL allows cost savings of up to 30% without decreasing EV yield (Figure 5A-C).
- Please note that pre-concentration is not needed for urine samples of less than 2 mL (Figure 5B). Additionally, pre-concentrating urine samples does not decrease EV yield as the data show that EV recovery increases proportionally with input urine volume (Figure 5C).

Figure 3. EasySep™ Can Be Used to Isolate EVs from Both Fresh and Frozen Urine
Fresh and frozen urine samples were pre-processed as described in the Sample Processing Protocol. Then, EVs were isolated according to the Protocol for EasySep™ EV Isolation from Urine. Each sample was obtained from 8 mL of input urine volume. Following EV isolation, 250 µL of PBS was added to resuspend each pre-concentrated sample and normalize them all to equal volumes. Next, 35 µL of sample was loaded per lane and analyzed by western blot. Results show that EVs isolated from both fresh and frozen urine express all three tetraspanins markers (CD9, CD63, and CD81) and low contaminant (THP) levels. Therefore, the data confirm that the Protocol for EasySep™ EV Isolation from Urine is compatible with both fresh and frozen urine samples.
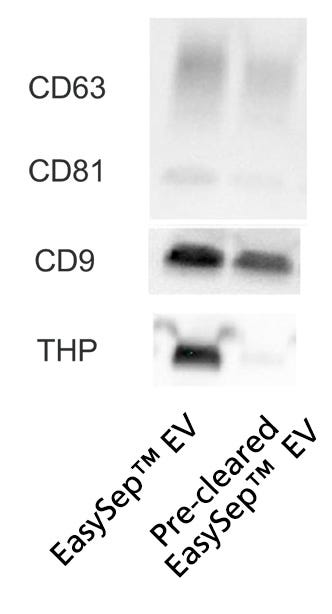
Figure 4. Pre-Clearing Urine Samples Increases EV Purity While Lowering THP Contamination
EVs were isolated from pre-cleared and normal (non-pre-cleared) urine samples. Then, recovery performance and contamination were compared. Pre-clearing and EV isolation were performed as described below in the Sample Processing Protocol and Protocol for EasySep™ EV Isolation from Urine, respectively. Each sample was obtained from 2 mL of input urine. Following EV isolation, 250 µL of PBS was added to resuspend each pre-concentrated sample and normalize them all to equal volumes. Next, 35 µL of sample was loaded per lane and analyzed by western blot to compare EV recovery through tetraspanin (CD9, CD63, and CD81) expression and contamination through THP urinary protein levels. Pre-clearing reduced THP contamination levels in isolated EVs with minor loss in EV yield.
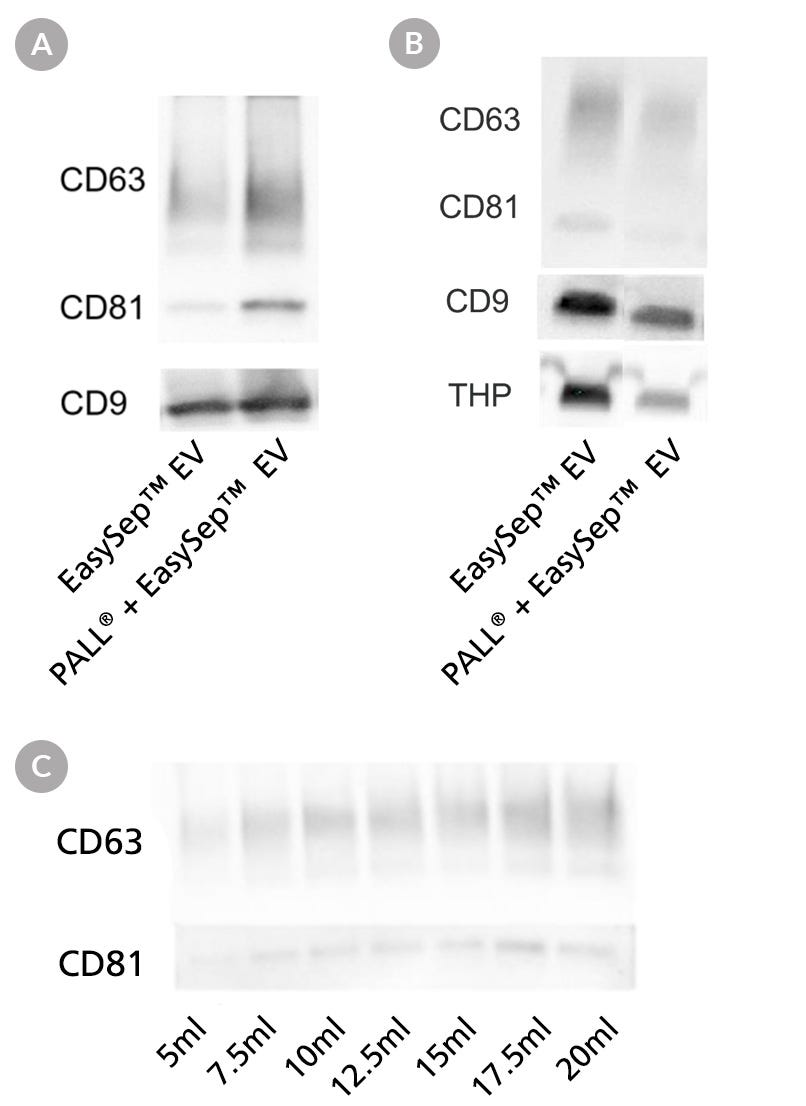
Figure 5. Urine Pre-Concentration Allows Processing of Large-Volume Samples for EV Isolation with EasySep™
EVs were isolated from pre-concentrated and normal (non-pre-concentrated) urine samples using the Protocol for EasySep™ EV Isolation from Urine. A. EVs were isolated either directly from 8 mL of urine, or from 1 mL of pre-concentrated urine following PALL® filtration of 8 mL of urine. Western blot analysis showed similar expression of EV tetraspanins in both urine samples, which suggests pre-concentrating urine samples does not affect EV recovery. B. EVs were isolated either directly from 2 mL of urine or from 1 mL of pre-concentrated urine following PALL® filtration of 2 mL of urine. After EV isolation, 250 µL of PBS was added to resuspend each pre-concentrated sample and normalize them all to equal volumes. Next, 35 µL of sample was loaded per lane and analyzed by western blot. Results show similar expression of EV tetraspanins in both urine samples, suggesting that concentrating urine samples when the volume is 2 mL or lower is not necessary. C. EVs were isolated from 5, 7.5, 10, 12.5, 15, 17.5, and 20 mL of normal urine (from left to right). First, all samples were pre-concentrated using PALL® filtration prior to EV isolation, as described in the Protocol for EasySep™ EV Isolation from Urine. Following EV isolation, 250 µL of PBS was added to resuspend each pre-concentrated sample and normalize them all to equal volumes. Next, 35 µL of sample was loaded per lane for western blot analysis. Results show that the expression of EV tetraspanins increases proportionally with input urine volume, which suggests pre-concentrating urine samples does not affect EV recovery.
Sample Processing Protocol
To pre-process fresh urine for EV isolation, follow the steps as described below. To freeze samples, follow steps 1 through 4. To use frozen urine, thaw fully before use, repeat steps 1 and 2, and proceed to step 5 for optional pre-clearing.
- Vortex urine sample to obtain a homogeneous suspension.
- Centrifuge urine at 1000 x g for 10 minutes. Transfer supernatant to a new tube.
- Optional: Add protease inhibitors to prevent protein degradation.
- Proceed to step 5 or freeze urine samples as per latest ISEV or laboratory guidelines (e.g. van Royen et al).7
- Optional: Centrifuge supernatant at 10,000 x g for 30 minutes to pre-clear sample. Transfer supernatant to the required tube. Note: This pre-clearing step reduces THP contamination but may result in lower EV recovery (see Figure 4).
Protocol for EasySep™ EV Isolation from Urine
The following protocol was optimized to isolate EVs from pre-concentrated samples prepared from large volumes of urine (2 - 20 mL). For samples of normal urine (i.e. non-pre-concentrated), please refer to the EasySep™ Human Pan-Extracellular Vesicle Positive Selection Kit Product Information Sheet for further details. This protocol may be used to isolate individual EV subpopulations using the EasySep™ single tetraspanin kits for CD9 (Catalog #17894), CD63 (Catalog #17895), or CD81 (Catalog #17892); in addition to the Pan-EV kit (Catalog #17891).
- Add urine sample (> 2 to 20 mL) to a 100K centrifugal filter tube, e.g. PALL® filter.
- Centrifuge at 1000 x g for 30 minutes at room temperature (15 - 25°C).
- Collect retained volume above filter membrane and top volume up to 1 mL with D-PBS (Without Ca++ and Mg++; Catalog #37350) to reconstitute the concentrated sample.
- Transfer sample to a 5 mL (12 x 75 mm) polystyrene round-bottom tube (e.g. Catalog #38007).
- Add 50 µL of Selection Cocktail per 1 mL of sample (e.g. for a 2 mL sample, add 100 µL of Selection Cocktail).
- Mix and incubate at room temperature for 30 minutes.
- Vortex Releasable RapidSpheres™ for 30 seconds.
Note: Particles should appear evenly dispersed - Add 100 µL of Releasable RapidSpheres™ per 1 mL of sample: (e.g. for a 2 mL sample, add 200 µL of Releasable RapidSpheres™).
- Mix and incubate for 30 minutes at room temperature.
- Add the D-PBS (Without Ca++ and Mg++; Catalog #37350) to top up the sample to 2.5 mL and mix by gently pipetting up and down 2 - 3 times.
- Place the tube (without lid) into the magnet (Catalog #18000) and incubate at room temperature for 5 minutes.
- Pick up the magnet, and in one continuous motion, invert the magnet and tube to pour off and discard the supernatant.
Note: Do not remove the tube from the magnet between separations - Repeat step 10.
- Incubate the sample at room temperature for 1 minute.
- Repeat steps 12 - 14 two more times.
- Remove the tube from the magnet and wash the sides with the desired medium to collect as many urine-derived EVs as possible.
- Isolated urine-derived EVs are now ready for use in downstream applications.
Product Information
| Product | Unit Size | Catalog # |
|---|---|---|
| EasySep™ Human Pan-Extracellular Vesicle Positive Selection Kit | 1 Kit | 17891 |
| EasySep™ Human Extracellular Vesicle (CD81) Positive Selection Kit | 1 Kit | 17892 |
| EasySep™ Human Extracellular Vesicle (CD9) Positive Selection Kit | 1 Kit | 17894 |
| EasySep™ Human Extracellular Vesicle (CD63) Positive Selection Kit | 1 Kit | 17895 |
| EasySep™ Extracellular Vesicle PE Positive Selection Kit | 1 Kit | 100-0812 |
| EasySep™ Magnet | 1 Unit | 18000 |
| Falcon® Round-Bottom Polystyrene Tubes, 5 mL | 500 Tubes | 38007 |
| Extracellular Vesicle Human CD9/CD63/CD81 Antibody Panel | 1 Kit | 100-0211 |
| RoboSep™-16 | 1 Unit | 23000 |
References
- Théry C et al. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1): 1535750.
- Pisitkun T et al. (2006) Discovery of urinary biomarkers. MCP 5(10): 1760–71.
- Miranda C et al. (2010) Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int 78(2): 191–9.
- Gámez-Valero A et al. (2015) Urinary extracellular vesicles as source of biomarkers in kidney diseases. Front Immunol 6(6).
- Trnka P et al. (2012) Urinary biomarkers in obstructive nephropathy. Clin J Am Soc Nephrol 7(10): 1567–75.
- Erdbrügger U et al. (2021) Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J Extracell Vesicles 10(7): e12093.
- van Royen ME et al. (2023) The quick reference card "Storage of urinary EVs" - A practical guideline tool for research and clinical laboratories. J Extracell Vesicles 12(3): e12286.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration


