Generation of Natural Killer Cells from Human Pluripotent Stem Cells Using STEMdiff™ and StemSpan™ Media and Supplements
- Document # 27195
- Version 1.0.0
- Jul 2021
Introduction
Natural killer (NK) cells are lymphocytes that provide defense against pathogens and tumors. NK cells play a critical role in innate immunity due to their secretion of proinflammatory cytokines and their ability to kill cancerous or virus-infected cells. While NK cells can be isolated from peripheral blood, human pluripotent stem cells (hPSCs) offer a potentially unlimited source. Methods to promote differentiation of hPSCs to NK cells provides useful tools for research into the basic biology of these cells and for developing adoptive immunotherapy for cancer treatment.
STEMdiff™ NK Cell Kit (Catalog #100-0170) facilitates the differentiation of hPSCs to NK cells, without the use of stromal cells or serum.
Differentiate hPSCs to NK Cells
STEMdiff™ NK Cell Kit comprises STEMdiff™ Hematopoietic - EB reagents paired with StemSpan™ NK Cell Generation Kit. STEMdiff™ NK Cell Kit is used in a four-stage protocol to differentiate hPSCs to NK cells. In the first two stages, STEMdiff™ Hematopoietic - EB reagents are used to differentiate hPSCs to CD34+ hematopoietic progenitor cells (Figure 1). First, hPSCs are cultured for 3 days in EB Formation Medium and EB Medium A to induce mesoderm specification. This is done in an AggreWell™ plate, allowing for the formation of uniformly sized embryoid bodies (EBs). In the second stage, the cells are cultured for 9 days in EB Medium B, promoting their specification to hematopoietic progenitor cells. The generated EBs are then dissociated into single cells, and CD34+ cells are enriched using EasySep™ immunomagnetic cell isolation.
In the third stage, CD34+ cells are cultured for 14 days in StemSpan™ Lymphoid Progenitor Expansion Medium on plates coated with StemSpan™ Lymphoid Differentiation Coating Material to stimulate their proliferation and differentiation to lymphoid progenitor (LP) cells. Finally, LP cells are differentiated to CD56+ NK cells (Figure 2).
For NK cell differentiation, the LP cells are cultured for 14 days in non-coated plates in StemSpan™ NK Cell Differentiation Medium containing the small molecule UM729 (Catalog #72332; sold separately) to promote their further differentiation to CD56+ NK cells. UM729 is necessary during this last stage of the culture to achieve high NK cell frequencies and yields. In this system, approximately 230 NK cells can be generated per CD34+ cell (average of results from multiple embryonic stem [ES]/induced pluripotent stem [iPS] cell lines; see Figure 5).
Why Use STEMdiff™ for Generating NK Cells?
- Eliminate variation introduced by serum and stromal cell lines by culturing cells in serum- and feeder-free conditions.
- Reduce variability by producing uniform aggregates for EB formation with AggreWell™.
- Produce approximately 230 NK cells per input hPSC-derived CD34+ cell.
- Avoid extra passaging steps required with stromal cell-based cultures.
STEMdiff™ NK Cell Kit Components
The following products are components of STEMdiff™ NK Cell Kit (Catalog #100-0170) and are also available for individual sale.
| Component | Quantity | Catalog # |
|---|---|---|
| STEMdiff™ Hematopoietic - EB Basal Medium | 120 mL | 100-0171 |
| STEMdiff™ Hematopoietic - EB Supplement A | 265 μL | 100-0172 |
| STEMdiff™ Hematopoietic - EB Supplement B | 7 mL | 100-0173 |
| StemSpan™ SFEM II | 100 mL* | 09605 |
| StemSpan™ Lymphoid Progenitor Expansion Supplement (10X) | 5 mL | 09915 |
| StemSpan™ Lymphoid Differentiation Coating Material (100X) | 250 μL | 09925 |
| StemSpan™ NK Cell Differentiation Supplement (100X) | 500 μL | 09950 |
*500 mL format is also available (Catalog #09655).
Note: The small molecule UM729 (Catalog #72332) is required for optimal differentiation of NK cells using STEMdiff™ NK Cell Kit. This product is not included in the kit and must be purchased separately.
Protocol for Differentiation of hPSCs to NK Cells
This protocol is designed to promote the differentiation of hPSCs to CD56+ NK cells over 40 days of culture. The first two stages—mesoderm formation and hematopoietic specification—are shown in Figure 1. Stage three—lymphoid progenitor differentiation—and stage four—NK cell differentiation—are shown in Figure 2. Please refer to the Technical Manual (Document #10000007537) for complete instructions.
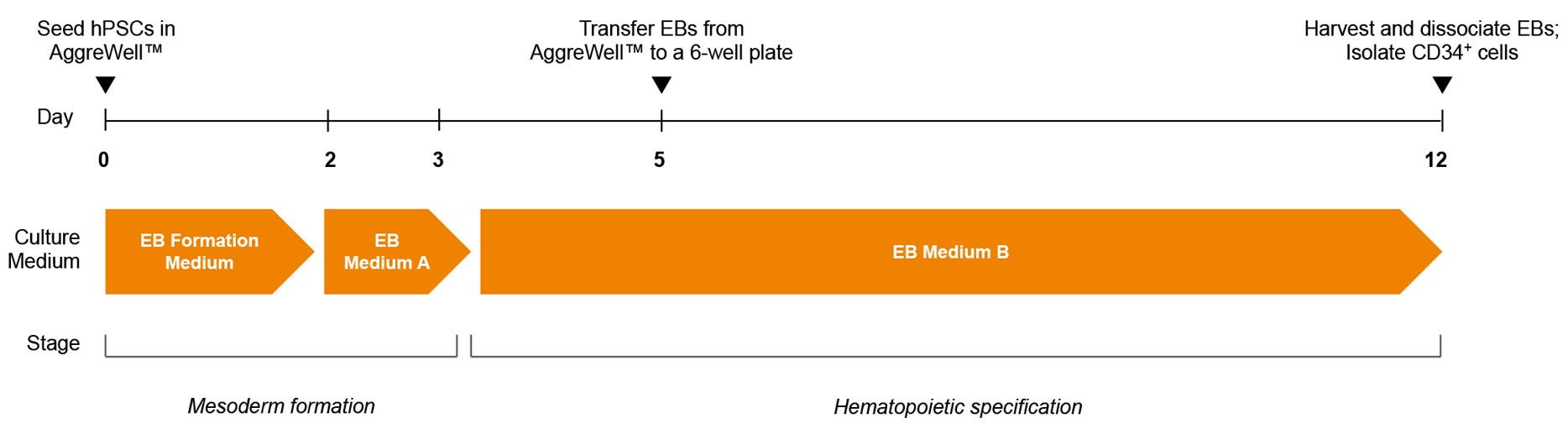
Figure 1. STEMdiff™ Hematopoietic - EB Progenitor Differentiation Protocol
hPSCs are harvested and dissociated into a single-cell suspension prior to seeding into AggreWell™ plates in EB Formation Medium (EB Medium A + 10 μM Y-27632) to form 600-cell aggregates. A half-medium change with EB Medium A is performed on day 2. After a total of 3 days of mesoderm formation, the medium is changed to EB Medium B to induce hematopoietic lineage differentiation. On day 5, EBs are transferred onto non-tissue culture-treated plates. After a total of 12 days, EBs are harvested and dissociated, then CD34+ are enriched by EasySep™ positive selection.
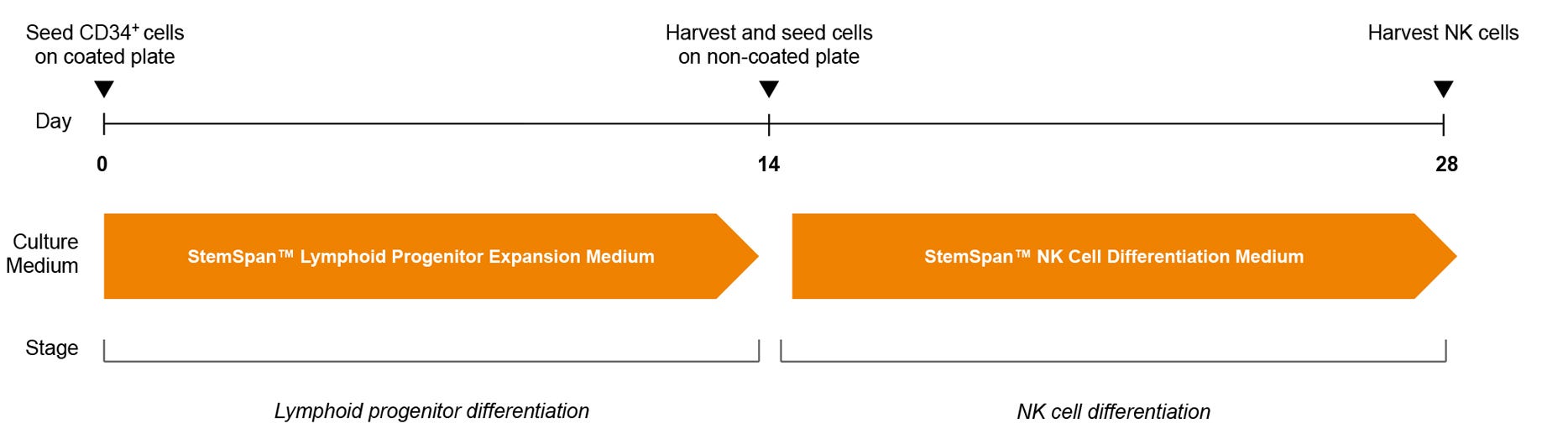
Figure 2. NK Cell Generation Protocol
hPSC-derived CD34+ cells are seeded in StemSpan™ Lymphoid Progenitor Expansion Medium on plates coated with StemSpan™ Lymphoid Differentiation Coating Material. On day 14, cells at the lymphoid progenitor stage are harvested and reseeded in StemSpan™ NK Cell Differentiation Medium onto non-coated plates for further differentiation to NK cells.
Note: UM729 should only be added to StemSpan™ NK Cell Differentiation Medium (see Step 17 of the protocol), but not StemSpan™ Lymphoid Progenitor Expansion Medium. NK cells are harvested after 28 days. For further details, see the step-by-step protocol on page 3.
Protocol
This procedure has been optimized for use with ES and iPS cells; refer to the Technical Manual (Document #10000007537) for complete instructions. Optimal cell yields depend on maintenance of cell health, which largely depends on following the recommended schedule of feeding and medium changes.
Differentiation of hPSCs to CD34+ Hematopoeitic Progenitor Cells (Stages 1 and 2)
Day 0
- Prepare EB Medium A (STEMdiff™ Hematopoietic - EB Basal Medium + STEMdiff™ Hematopoietic - EB Supplement A). Prepare EB Formation Medium by adding Y-27632 at 10 μM to EB Medium A.
- Prepare an AggreWell™400 plate by rinsing with Anti-Adherence Rinsing Solution, washing with DMEM/F-12 with 15 mM HEPES, and adding 2.5 mL of EB Formation Medium (per one well of a 6-well plate).
- Harvest hPSCs and generate a single-cell suspension using ACCUTASE™.
- Dilute hPSCs to 1.4 x 106 cells/mL in 2.5 mL of EB Formation Medium, then seed into the AggreWell™ plate that was prepared in step 2. Incubate at 37°C for 2 days.
Day 2
- Perform a half-medium change with EB Medium A. Incubate at 37°C for 24 hours.
Day 3
- Prepare EB Medium B (STEMdiff™ Hematopoietic - EB Basal Medium + STEMdiff™ Hematopoietic - EB Supplement B). Perform a half-medium change with EB Medium B. Incubate at 37°C for 2 days.
Day 5
- Harvest EBs, then filter and elute these with 2.5 mL EB Medium B using a 37 μm reversible strainer.
- Transfer eluted EBs to a non-tissue culture-treated plate. Incubate at 37°C for 2 days.
Day 7
- Add 2.5 mL EB Medium B. Incubate at 37°C for 3 days.
Day 10
- Perform a half-medium change with EB Medium B. Incubate at 37°C for 2 days.
Day 12
- Harvest EBs and dissociate into a single-cell suspension using Collagenase Type II and TrypLE™ Express. Isolate CD34+ cells using EasySep™ Human CD34 Positive Selection Kit II.
Differentiation of CD34+ Cells to CD56+ NK Cells (Stages 3 and 4)
Day 0
- Coat non-tissue culture-treated plates with StemSpan™ Lymphoid Differentiation Coating Material. Refer to the Technical Manual (Document #10000007537) for complete instructions.
- Prepare StemSpan™ Lymphoid Progenitor Expansion Medium (StemSpan™ SFEM II + StemSpan™ Lymphoid Progenitor Expansion Supplement).
- Dilute CD34+ cells (prepared in step 11) to 5 x 104 cells/mL in StemSpan™ Lymphoid Progenitor Expansion Medium and seed onto the coated plate (prepared in step 12).
- Incubate at 37°C for 14 days, following instructions in the manual for required half-medium changes and plate transfer on day 7.
Day 14
- Harvest lymphoid progenitor cells (containing CD5+CD7+ cells; see Figure 4) for further differentiation to NK cells.
- Prepare StemSpan™ NK Cell Differentiation Medium (StemSpan™ SFEM II + StemSpan™ NK Cell Differentiation Supplement + UM729*).
- Dilute lymphoid progenitor cells to 1 x 105 cells/mL in StemSpan™ NK Cell Differentiation Medium. Seed onto a non-coated tissue culture plate, incubate at 37°C, and follow instructions in the manual for required half-medium changes and additions.
Day 28
- Harvest cells containing CD56+ NK cells (see Figures 5 - 6) for use in downstream assays.
*UM729 purchased separately.
Analysis of Differentiated NK Cells
hPSCs are first differentiated to CD34+ hematopoietic progenitor cells over 12 days, which can be identified by flow cytometry (Figure 3A & 3B). The average frequency and yield of CD34+ cells are shown in Figure 3C. CD7+CD5+ lymphoid progenitor cells generated during 14 days of culture of hPSC-derived CD34+ cells (Figure 4) and CD56+ NK cells generated during an additional 14 days of culture (Figures 5 & 6) are identified by flow cytometry. Expression of other NK-specific cell surface markers, such as NKp46, NKp44, NKp30, NKG2D, and CD16 are also examined (Figures 5A, 5B, & 6). In the examples in Figure 6, staining for killer-cell immunoglobulin-like receptor (KIR) molecules was performed using one or two different antibodies: HP-MA4, or HP-MA4 and 180704, which recognize distinct KIR molecules. The average frequency and yield of the indicated hematopoietic progenitor, lymphoid progenitor, and NK cell populations generated in culture, are shown in Figures 3C, 4C, and 5C. hPSC-derived NK cells are functionally similar to NK cells purified from peripheral blood as measured using a cytotoxicity assay (Figure 7) and by assessment of degranulation and cytokine production (Figure 8).
Applications for STEMdiff™ NK Cell Kit
- Research the differentiation of hPSCs to NK cells
- Assess the efficacy and toxicity of candidate therapeutics on NK cells during drug development
- Research the use of NK cells for potential development of cellular therapeutics
- Develop in vitro models to study diseases that involve NK cells
- Perform gene editing of progenitors prior to differentiation to NK cells
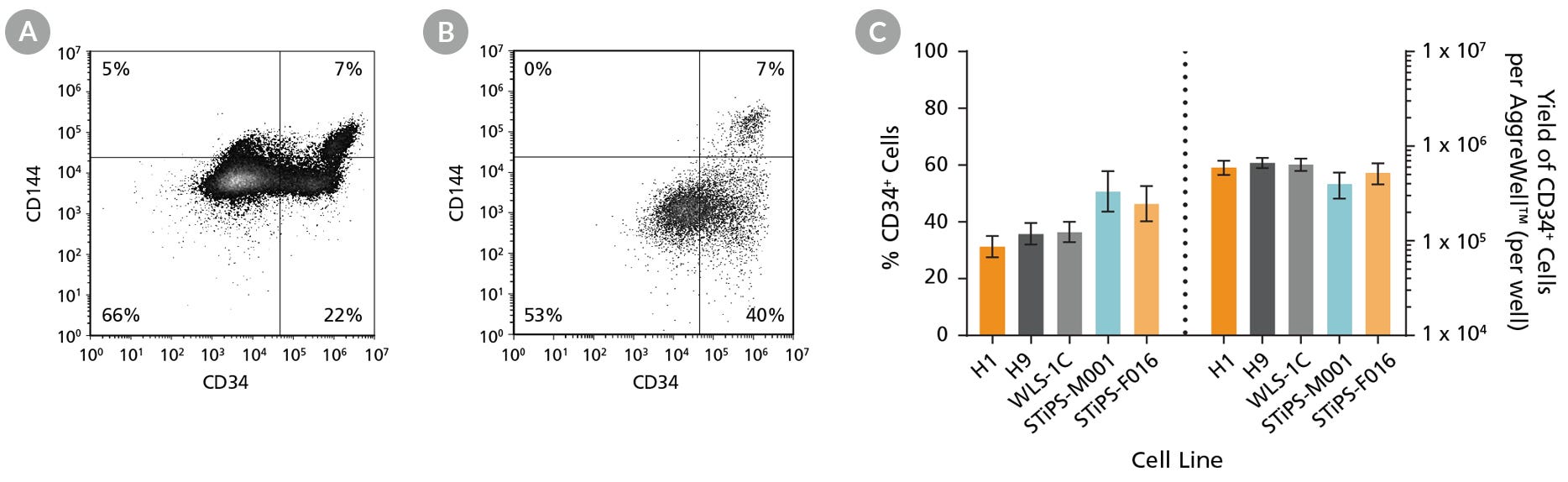
Figure 3. hPSCs Differentiate to CD34+ Hematopoietic Progenitor Cells After 12 Days of Culture
Human ES and iPS cells were induced to differentiate to CD34+ cells using the 12-day protocol shown in Figure 1. At the end of the culture period, cells were harvested, dissociated into a single-cell suspension, and analyzed by flow cytometry for CD34 and CD144 expression. Dead cells were excluded by light-scatter profile and DRAQ7™ staining. Representative flow cytometry plots for (A) ES (H1) cell-derived and (B) iPS (STiPS-M001) cell-derived cells analyzed on day 12 are shown. (C) The average frequency of viable CD34+ cells on day 12 (before CD34+ cell isolation) for two ES cell lines (H1 and H9) and three iPS cell lines (WLS-1C, STiPS-M001, and STiPS-F016) ranged between 31% and 51%. The average yield of CD34+ cells produced per well of a 6-well AggreWell™400 plate ranged between 4.0 x 105 and 6.7 x 105. Data are shown as mean ± SEM (n = 9 - 35).
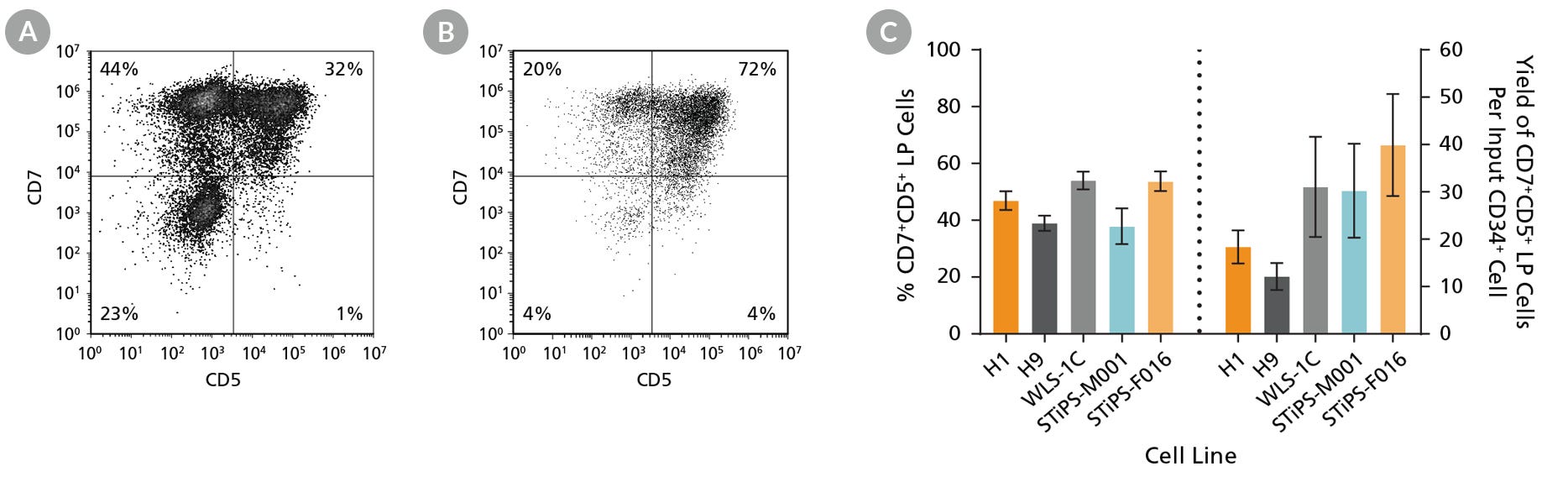
Figure 4. hPSC-Derived CD34+ Cells Differentiate to CD5+CD7+ Lymphoid Progenitor Cells Over 14 Days of Culture
hPSC-derived CD34+ cells were cultured for 14 days in StemSpan™ Lymphoid Progenitor Expansion Medium on plates coated with StemSpan™ Lymphoid Differentiation Coating Material (Figure 2). Cells were harvested and analyzed for CD7 and CD5 expression by flow cytometry. Representative flow cytometry plots for (A) ES (H1) cell-derived and (B) iPS (STiPS-M001) cell-derived cells are shown. (C) The average frequency of viable CD7+CD5+ lymphoid progenitor cells on day 14 ranged between 38% and 54%, and the average yield of lymphoid progenitor cells produced per input hPSC-derived CD34+ cell was between 12 and 40. Data are shown as mean ± SEM (n = 8 - 32).
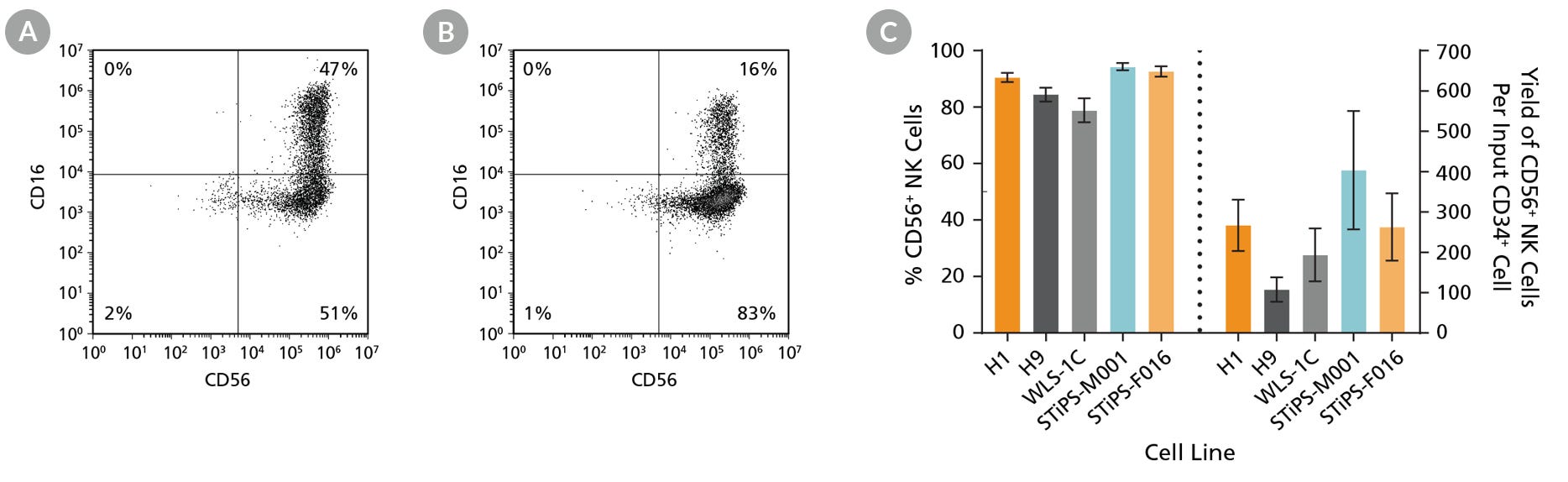
Figure 5. hPSC-Derived Lymphoid Progenitor Cells Differentiate to CD56+ NK Cells After 14 Days of Culture
hPSC-derived lymphoid progenitor cells were cultured in StemSpan™ NK Cell Differentiation Medium on non-coated plates for 14 days (Figure 2). Cells were harvested and analyzed for expression of CD56 and CD16 by flow cytometry. Representative flow cytometry plots are shown for both (A) ES (H1) cell-derived and (B) iPS (STiPS-M001) cell-derived cells. (C) After 28 days of culture, the average frequency of viable CD56+ NK cells from hPSC-derived CD34+ cells ranged between 79% and 94%. The average yield of CD56+ cells produced per hPSC-derived CD34+ cell was between 108 and 404. Data are shown as mean ± SEM (n = 7 - 18).
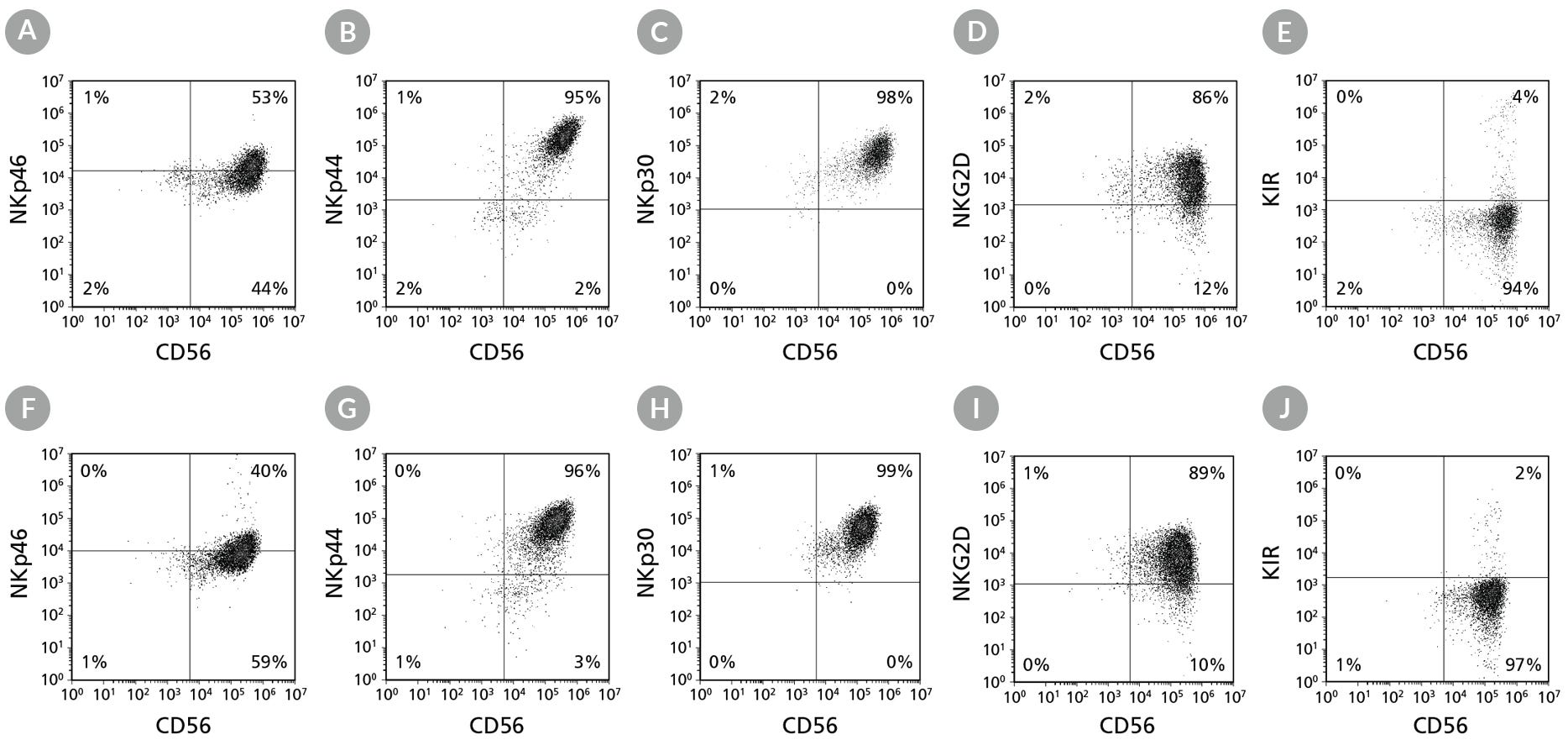
Figure 6. Cell Surface Marker Expression on hPSC-Derived CD56+ NK Cells After 28 Days of Culture
hPSC-derived CD34+ cells were cultured in StemSpan™ Lymphoid Progenitor Expansion Medium on plates coated with StemSpan™ Lymphoid Differentiation Coating Material for 14 days, followed by 14 days of culture in StemSpan™ NK Cell Differentiation Medium on non-coated plates to generate CD56+ NK cells (Figure 2). Cells were harvested and analyzed for CD56, NKp46, NKp44, NKp30, NKG2D, and KIR expression by flow cytometry. Dead cells were excluded by light-scatter profile and DRAQ7™ staining. Data shown are from representative cultures initiated with (A-E) ES (H1) cells or (F-J) iPS (STiPS-M001) cells.
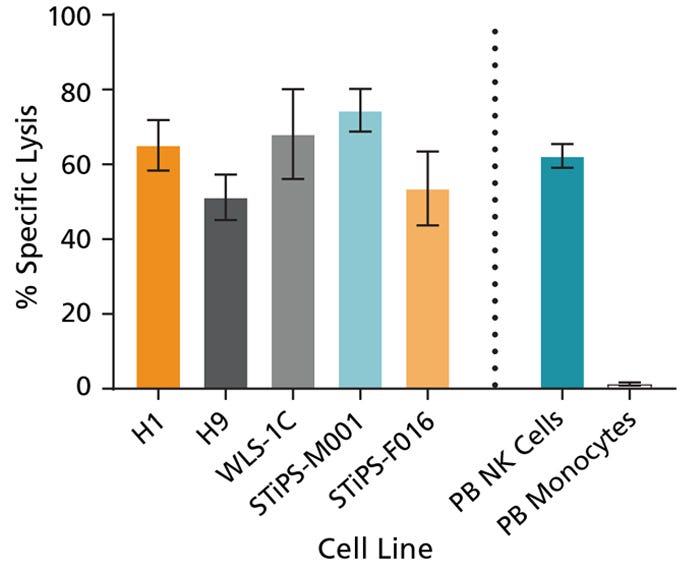
Figure 7. Cultured hPSC-Derived NK Cells Exhibit Cytotoxicity Toward K562 Cell Line
hPSC-derived CD56+ NK cells were co-cultured with calcein AM (CAM)-labeled K562 cells at a ratio of 2.5:1 for 4 hours. Isolated peripheral blood (PB) NK cells and monocytes were also co-cultured with labeled K562 cells as positive and negative controls, respectively. Before the co-culture, frozen PB NK cells were thawed and cultured overnight with StemSpan™ NK Cell Differentiation Supplement and StemSpan™ SFEM II, while PB monocytes were cultured overnight in SFEM II only. To measure maximum release, the labeled K562 cells were treated with 1% Triton™ X-100. Culture supernatants were assessed for fluorescence released by dead cells after 4 hours using a SpectraMax® microplate reader (excitation 485 nm/emission 520 nm). The % specific lysis was calculated as follows: [(test release - spontaneous release) / (maximum release - spontaneous release)] x 100%. The average specific lysis by hPSC- derived NK cells ranged between 51% and 75% as compared to 62% specific lysis by PB NK cells. Cultures of ES (H1 and H9) and iPS (WLS-1C, STiPS-M001, and STiPS-F016) cells are shown. Data are shown as mean ± SEM (n = 3 - 7).
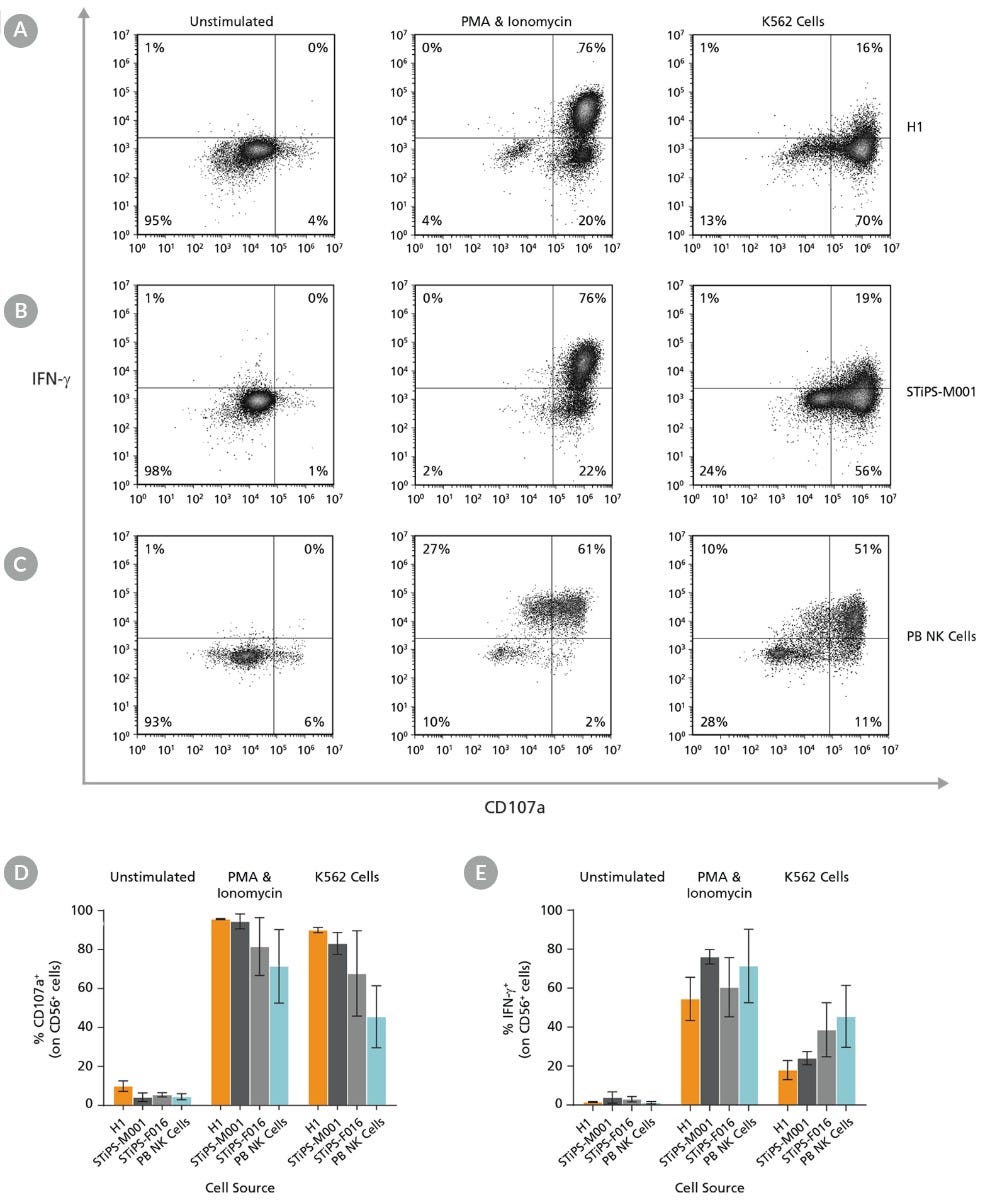
Figure 8. Cultured hPSC-Derived CD56+ NK Cells Are Induced to Express Surface CD107a and Produce IFN-γ After Co-Culture with K562 Target Cells or Stimulation with PMA and Ionomycin
hPSC-derived NK cells were obtained using STEMdiff™ NK Cell Kit as described. hPSC-derived CD56+ NK cells were left unstimulated or were stimulated with either phorbol 12-myristate 13-acetate (PMA) and ionomycin or with K562 cells at a ratio of 1:1 effector to target cells. CD107a antibody and stimulation factors were added to each well. After one hour, monensin and brefeldin A were added to each well to inhibit protein transport. After a total incubation time of 4 hours at 37°C, cells were washed and stained with Zombie NIR™ Fixable Viability Kit and CD56. Cells were then fixed, permeabilized, and stained for IFN-γ. Surface CD107a and intracellular IFN-γ expression were assessed using flow cytometry. Representative samples are gated on CD56. (A) Representative unstimulated, PMA & ionomycin- stimulated, and K562-stimulated ES (H1) cell-derived NK cell samples. (B) Representative unstimulated, PMA & ionomycin-stimulated, and K562-stimulated iPS (STiPS-M001) cell-derived NK cell samples. (C) Representative unstimulated, PMA & ionomycin-stimulated, and K562-stimulated PB NK cell samples. The PB NK cell sample was thawed and cultured overnight in StemSpan™ SFEM II supplemented with IL-2 prior to this assay. (D) Summary of CD107a and (E) IFN-γ expression results by NK cells. Upon stimulation, hPSC-derived CD56+ NK cells are able to degranulate, as shown by surface expression of CD107a (average range: 82 - 96% for PMA & ionomycin stimulation and 68 - 90% for K562 stimulation) and secrete IFN-γ (54 - 76% and 18 - 39% for PMA & ionomycin and K562 stimulation, respectively). Data are shown as mean ± SEM (n = 2 - 4 ).
Product Information
Recommended Antibodies for Analysis*
| Product Name | Catalog # |
|---|---|
| Anti-Human CD34 Antibody, Clone 581 | 60013 |
| Anti-Human CD5 Antibody, Clone UCHT2 | 60082 |
| Anti-Human CD7 Antibody, Clone CD7-6B7 | N/A |
| Anti-Human CD16 Antibody, Clone 3G8 | 60041 |
| Anti-Human CD56 Antibody, Clone HCD56 | 60021 |
| Anti-Human CD158 (KIR) Antibody, Clone 180704 and/or HP-MA4 | N/A |
| Anti-Human CD335 (NKp46) Antibody, Clone 9E2 | N/A |
| Anti-Human CD336 (NKp44) Antibody, Clone P44-8 | N/A |
| Anti-Human CD337 (NKp30) Antibody, Clone P30-15 | N/A |
| Anti-Human NKG2D Antibody, Clone 1D11 | N/A |
| Anti-Human IFNγ, Clone 4S.B3 | N/A |
| Anti-Human CD107a, Clone H4A3 | N/A |
*Not included in the kit.
Accessory Products*
| Product Name | Catalog # |
|---|---|
| UM729 | 72332 |
| Y-27632 | 72302 |
| AggreWell™400 6-Well (or 24-Well) Plate | 34421 (or 34411) |
| DMEM/F-12 with 15 mM HEPES | 36254 |
| Anti-Adherence Rinsing Solution | 07010 |
| ACCUTASE™ | 07920 |
| TrypLE™ Express | Thermo Fisher 12604013 |
| Collagenase Type II | 07418 |
| EasySep™ Human CD34 Positive Selection Kit II | 17856 |
| 37 μm Reversible Strainer, Large | 27250 |
| Calcein AM** | Sigma 56496 |
| Triton™ X-100** | Sigma X100 |
| Phorbol 12-Myristate 13-Acetate** | 74042 |
| Ionomycin** | 73722 |
| Brefeldin A** | 73012 |
| Monensin** | BioLegend 420701 |
*Not included in the kit.
**Additional products used to generate functional data for Figures 7 and 8.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration

