Organoid Culture Plates
Cell culture plates for easy and reproducible generation of organoids
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
-
 Gentle Cell Dissociation Reagent
Gentle Cell Dissociation ReagentcGMP, enzyme-free cell dissociation reagent
-
 DMEM/F-12 with 15 mM HEPES
DMEM/F-12 with 15 mM HEPESDulbecco's Modified Eagle's Medium/Nutrient Ham's Mixture F-12 (DMEM/F-12) with 15 mM HEPES buffer
-
 D-PBS (Without Ca++ and Mg++)
D-PBS (Without Ca++ and Mg++)Dulbecco’s phosphate-buffered saline without calcium and magnesium
-
 Y-27632 (Dihydrochloride)
Y-27632 (Dihydrochloride)RHO/ROCK pathway inhibitor; Inhibits ROCK1 and ROCK2
-
Labeling Antibodies
Compatible antibodies for purity assessment of isolated cells
Overview
The optimized well design reduces edge effects and eliminates the need for plate pre-warming, saving time and ensuring each well is usable, resulting in an improved performance from each experiment. Compatible with Corning® Matrigel® and other matrices, as well as automated systems, this plate fits perfectly into high-throughput workflows, making it an ideal solution for large-scale studies, such as drug screening or disease modeling. Organoids consistent in size, shape, growth, and maturation can help build physiologically relevant models more representative of in vivo environments, ideal for studying complex disease pathways, testing therapeutic responses, and advancing large-scale in vitro modeling.
Organoid Culture Plates can be used in most organoid workflows that are typically embedded in domes, including intestinal organoids grown with IntestiCult™ Organoid Growth Medium (Human) and IntestiCult™ Organoid Differentiation Medium (Human), or hepatic organoids grown with HepatiCult™ Organoid Growth Medium (Human) and HepatiCult™ Organoid Differentiation Medium (Human).
Data Figures
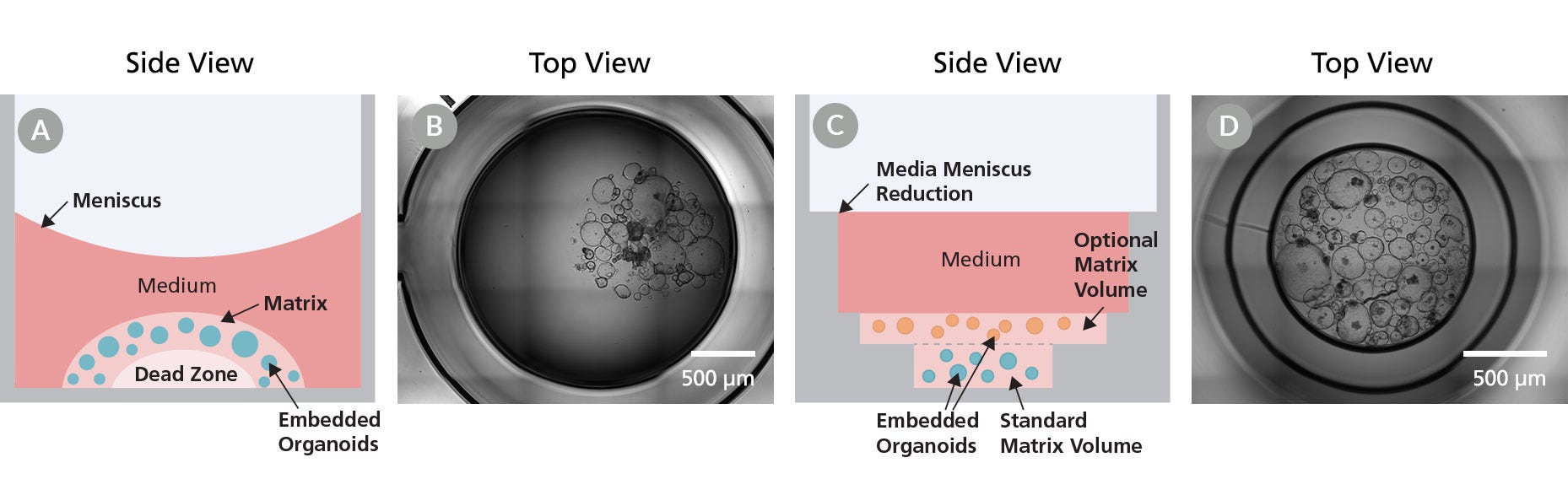
Figure 1. Organoid Culture Plates Enable Superior Imaging and Ease of Use Over Dome Culture in Standard Culture Plates
(A,B) Challenges associated with organoids cultured in matrix domes using standard culture plates include inconsistencies by manually placing domes in the center of the well, culture “dead zones” with poor or no growth, variability in dome shape, height, and position, the inability to form domes from diluted matrix, and imaging impairment due to the meniscus. (C,D) The stepped features of the Organoid Culture Plates support a standardized matrix depth, reduced meniscus, and increased culture robustness resulting in more uniform growth, uniform organoid position, and fewer failed wells. Additionally, an optional second matrix volume enables layered cultures or co-cultures. Taken together, Organoid Culture Plates enable increased user efficacy, as well as clearer imaging for more accurate observations to benefit detailed studies and both quantitative and qualitative data collection.
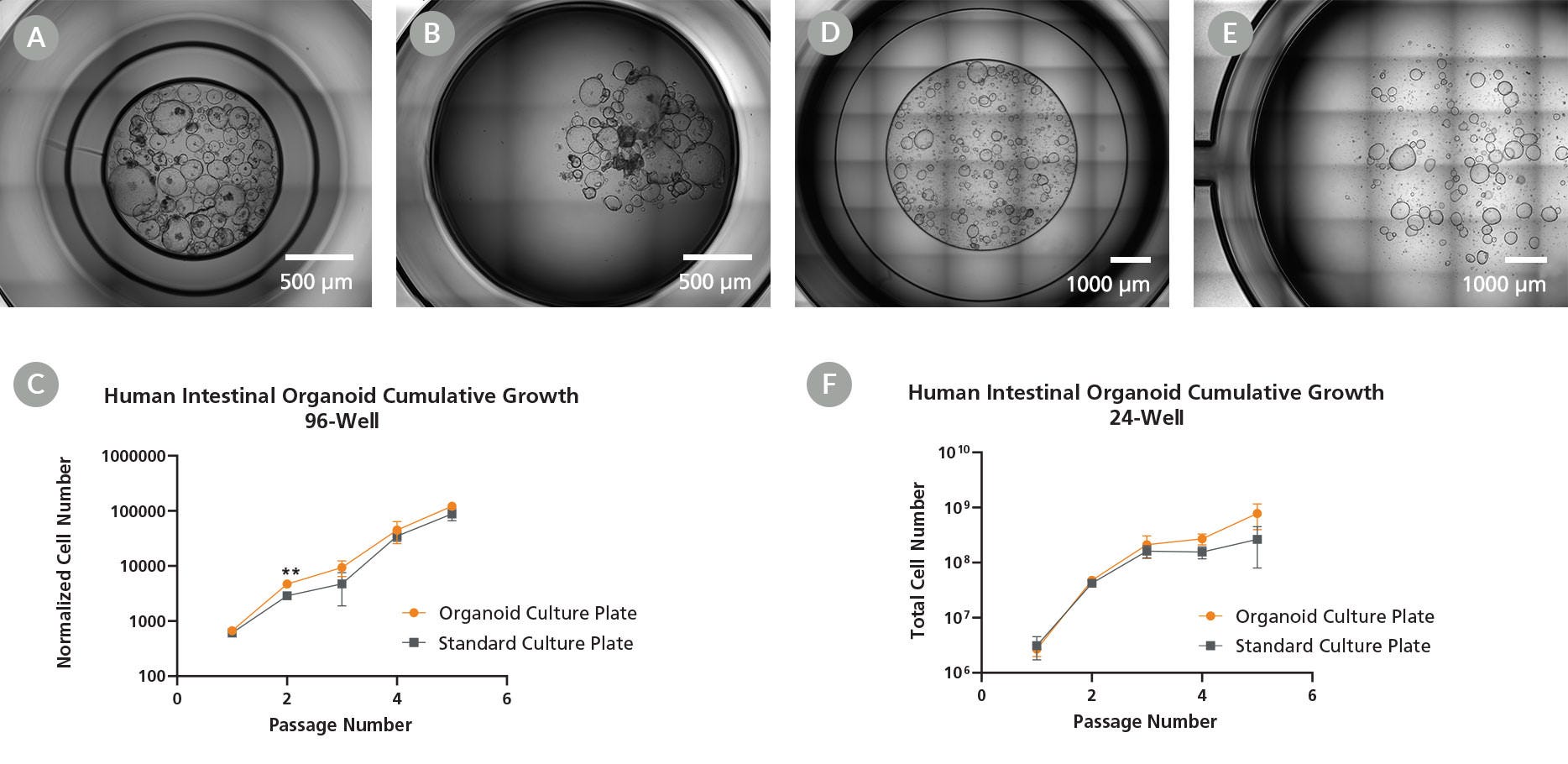
Figure 2. Organoid Culture Plates Support Equivalent Cell Growth in Addition to Enhanced Imaging
Organoid morphology and growth in the Organoid Culture Plates were assessed against dome culture in standard culture plates in both (A,B,C) 96- and (D,E,F) 24-well plate formats. Brightfield images show expected cystic morphology in human intestinal organoids cultured in the (A) 96-well Organoid Culture Plate and (B) 96-well standard culture plate after 7 days. (C) Human intestinal organoid growth was assessed in the 96-well Organoid Culture Plates or standard culture plates over 5 passages. The cell number was normalized to the number of fragments at Day 0 to account for different seeding volumes between the 96-well Organoid Culture Plates and the 96-well standard culture plates. The Organoid Culture Plates had a significantly higher normalized cell number at passage 2 but were otherwise equivalent in their capacity to support cellular growth when compared to standard culture plates (n = 3). Brightfield images show expected cystic morphology in human intestinal organoids culture in the (D) 24-well Organoid Culture Plate and (E) 24-well standard standard culture plates after 7 days. (F) Human intestinal organoid growth was assessed in the 24-well Organoid Culture Plates or in standard culture plates over 5 passages, demonstrating equivalent capacity to support cellular growth between culture plates (n = 3), ** p < 0.01 Significance determined using a mixed-effects analysis with Sidak’s test for multiple comparisons.
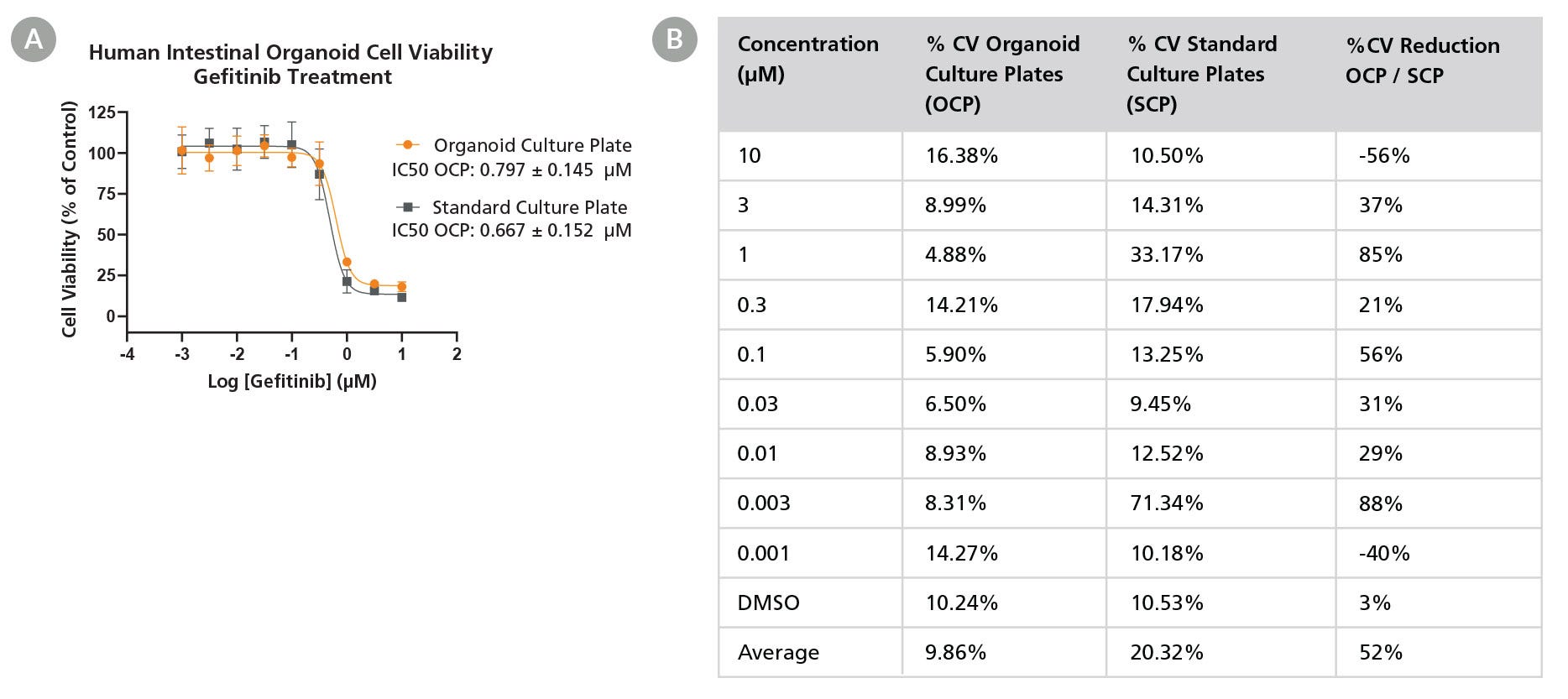
Figure 3. Organoid Culture Plates Enhance Consistency in Toxicity Screening by Providing a Standardized Platform
Human-derived intestinal organoids were cultured in 96-well Organoid Culture Plates or 96-well standard culture plates for 2 days, followed by Gefitinib treatment for an additional 4 days. Relative cell viability was assessed on Day 6 via CellTiterGloⓇ 3D analysis. (A) Screening cultures in Organoid Culture Plates produced similar IC50 values to organoid domes in standard culture plates after Gefitinib treatment (0.797 ± 0.145 µM and 0.667 ± 0.152 µM respectively). (B) Organoids cultured in the Organoid Culture Plates had a lower coefficient of variation in cell viability between technical replicates following the Gefitinib treatment compared to the dome cultures from the standard culture plates, resulting in higher confidence and more robust data. %CV = Percent coefficient of variation. OCP = Organoid Culture Plate. SCP = Standard Culture Plate. %CV reduction was calculated using 1 - %CV Organoid Culture Plate / %CV Standard Culture Plate
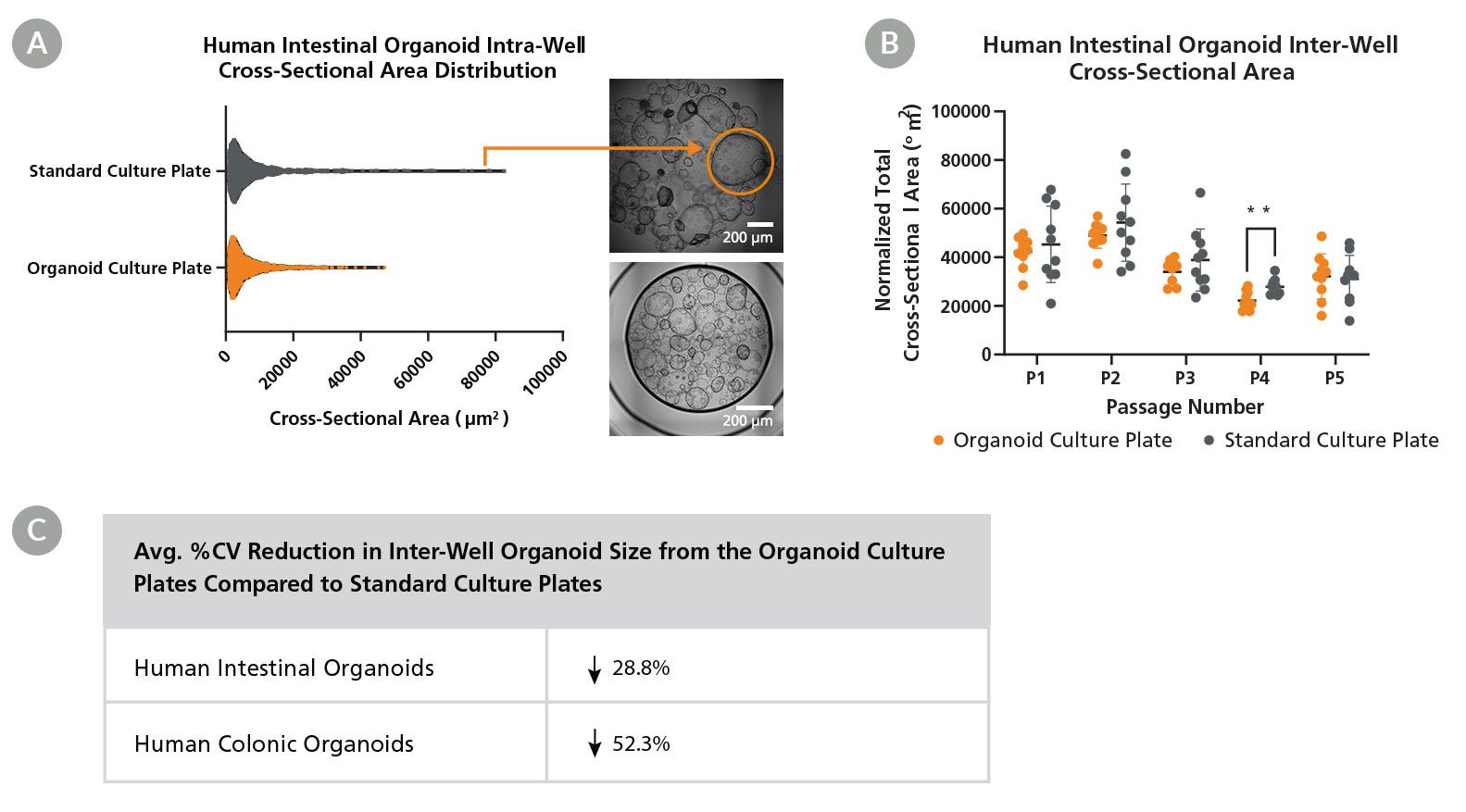
Figure 4. Organoid Culture Plates Reduce Intra-Well and Inter-Well Heterogeneity for Increased Consistency in Organoid Size
(A) Within a single well, human intestinal organoids cultured in the 96-well Organoid Culture Plates have a reduced range of organoid cross-sectional area when compared to human intestinal organoids cultured in standard culture plates. (B) For inter-well comparisons, organoids cultured in the Organoid Culture Plates exhibited lower variability in cross-sectional area compared to the organoid cultured in standard culture plates, as indicated by the reduced spread of the data. (C) The coefficient of variation (%CV) was used to measure consistency and reproducibility in the inter-well organoid cross-sectional area between culture plate formats, demonstrating reduced inter-well organoid size for organoids cultured in the Organoid Culture Plates. %CV reduction was calculated using 1 - %CV Organoid Culture Plate/%CV Standard Culture Plate
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Resources and Publications
Educational Materials (9)
Related Products
-
 IntestiCult™ Organoid Growth Medium (Human)
IntestiCult™ Organoid Growth Medium (Human)Cell culture medium for establishment and maintenance of human intestinal organoids
-
 CryoStor® CS10
CryoStor® CS10Animal component-free, defined cryopreservation medium with 10% DMSO
-
 IntestiCult™ Organoid Differentiation Mediu...
IntestiCult™ Organoid Differentiation Mediu...Cell culture medium for further differentiation of human intestinal organoids in 3D, or as monolayers/air-liquid interface cultures
-
 HepatiCult™ Organoid Kit (Human)
HepatiCult™ Organoid Kit (Human)Culture medium kit for initiation, growth, and differentiation of human liver organoids
Item added to your cart

Organoid Culture Plates
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.