HepatiCult™ Organoid Kit (Human)
Culture medium kit for initiation, growth, and differentiation of human liver organoids
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
-
 Trypan Blue
Trypan BlueReagent for counting viable mammalian cells
-
 Ammonium Chloride Solution
Ammonium Chloride SolutionReagent for lysis of red blood cells
-
 Costar® 24-Well Flat-Bottom Plate, Tissue Cu...
Costar® 24-Well Flat-Bottom Plate, Tissue Cu...Clear polystyrene flat-bottom, tissue culture-treated multiwell cell culture plate with lid
-
 Y-27632 (Dihydrochloride)
Y-27632 (Dihydrochloride)RHO/ROCK pathway inhibitor; Inhibits ROCK1 and ROCK2
-
Labeling Antibodies
Compatible antibodies for purity assessment of isolated cells
Overview
HepatiCult™ Organoid Kit (Human) contains all the necessary components to culture human liver organoids from fresh or cryopreserved human liver tissue:
• HepatiCult™ Organoid Initiation Medium (Human) for efficient generation of hepatic organoids across different donor lines
• HepatiCult™ Organoid Growth Medium (Human) for expansion and maintainence of hepatic organoids for future experimentation or bio-banking
• HepatiCult™ Organoid Differentiation Medium (Human) for generating mature organoids that demonstrate hepatic functionality, including CYP3A4 activity. These organoids can be adapted to a range of culture protocols, including 2D monolayer, suspension cultures, and high-throughput assays
For more information on protocols for organoid culture with HepatiCult™, please explore the Technical Manual and Educational Materials.
Should you intend to use this product for commercial purposes, please contact HUB at www.huborganoids.nl for a commercial use license or for clarifications in relation to HUB licensing.
Data Figures
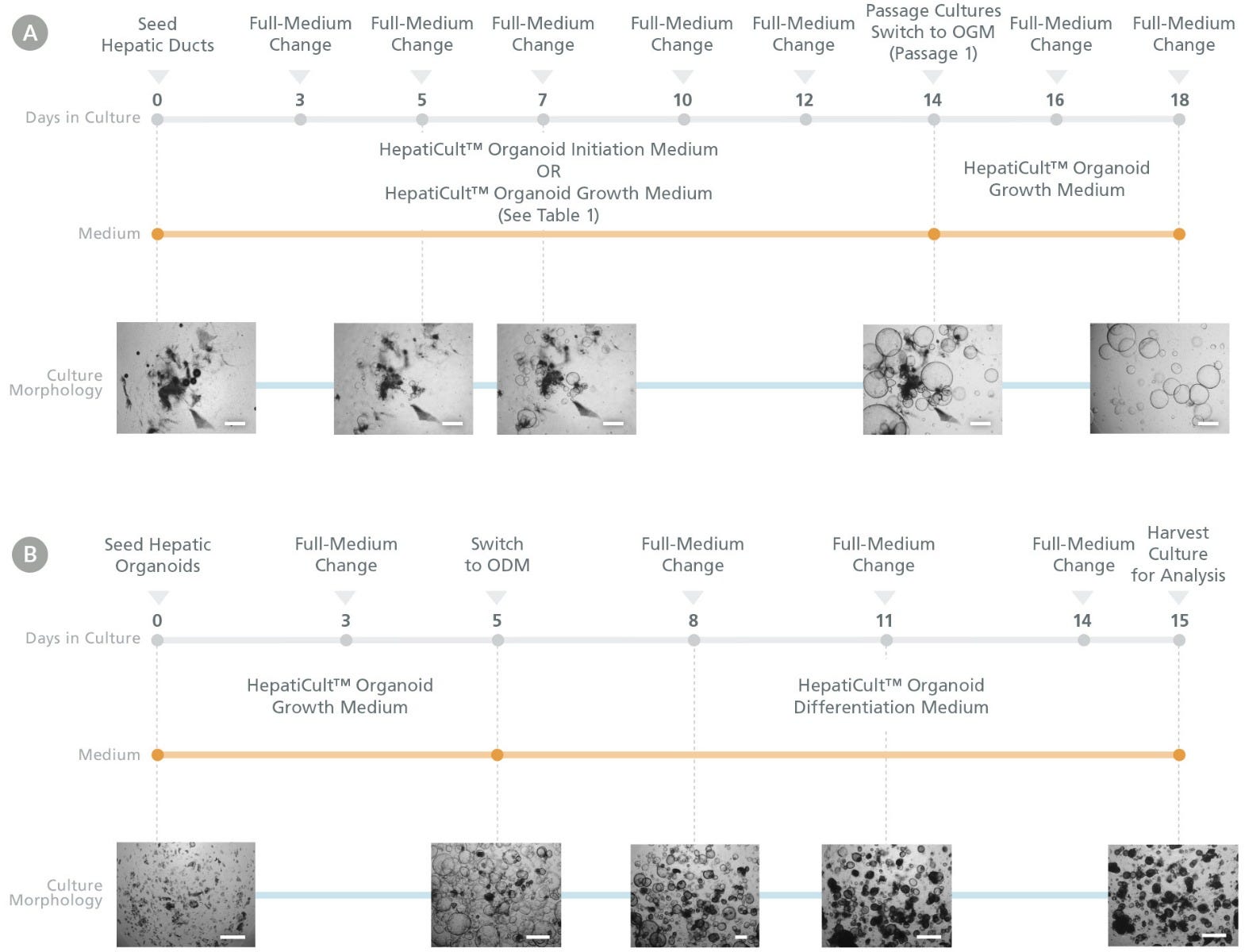
Figure 1. HepatiCult™ Organoid Kit (Human) Enables Liver Organoid Initiation, Expansion, and Differentiation
Human liver organoids can be grown from normal human liver tissue-derived hepatic ducts using the HepatiCult™ Organoid Kit (Human). (A) Cultures are established in HepatiCult™ Organoid Initiation Medium (OIM; Human) or HepatiCult™ Organoid Growth Medium (OGM; Human) (see Table 1 below) and subsequently passaged in HepatiCult™ OGM for expansion. (B) After passaging 2-3 times in HepatiCult™ OGM, cultures can be switched to HepatiCult™ Organoid Differentiation Medium (ODM; Human) to differentiate organoids towards more mature hepatic cell types. Refer to the Product Manual (Document #10000008301) for full culturing protocols.
Table 1. Product Recommendations for Liver Organoid Initiation, Expansion, and Differentiation
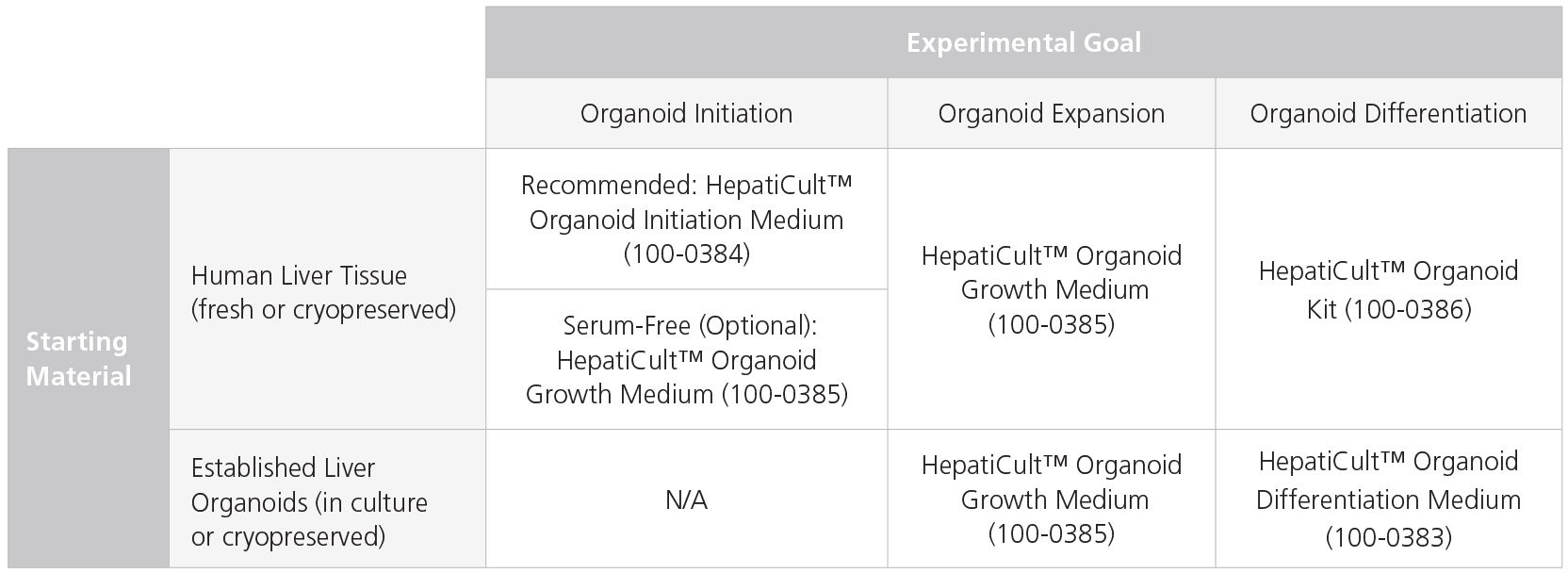
The recommended configuration of the HepatiCult™ Organoid Kit (Human) may differ based on starting material and experimental goals. When establishing liver organoid cultures from human liver tissue, HepatiCult™ Organoid Initiation Medium (OIM; Human) is recommended for efficient organoid initiation (see Figure 2 below). The expansion of already established organoids (fresh in culture or cryopreserved) is supported by HepatiCult™ Organoid Growth Medium (OGM; Human). These organoids should be maintained for 2-3 passages before further differentiation using HepatiCult™ Organoid Differentiation Medium (ODM). Refer to the Product Manual (Document #10000008301) for full culturing protocols.
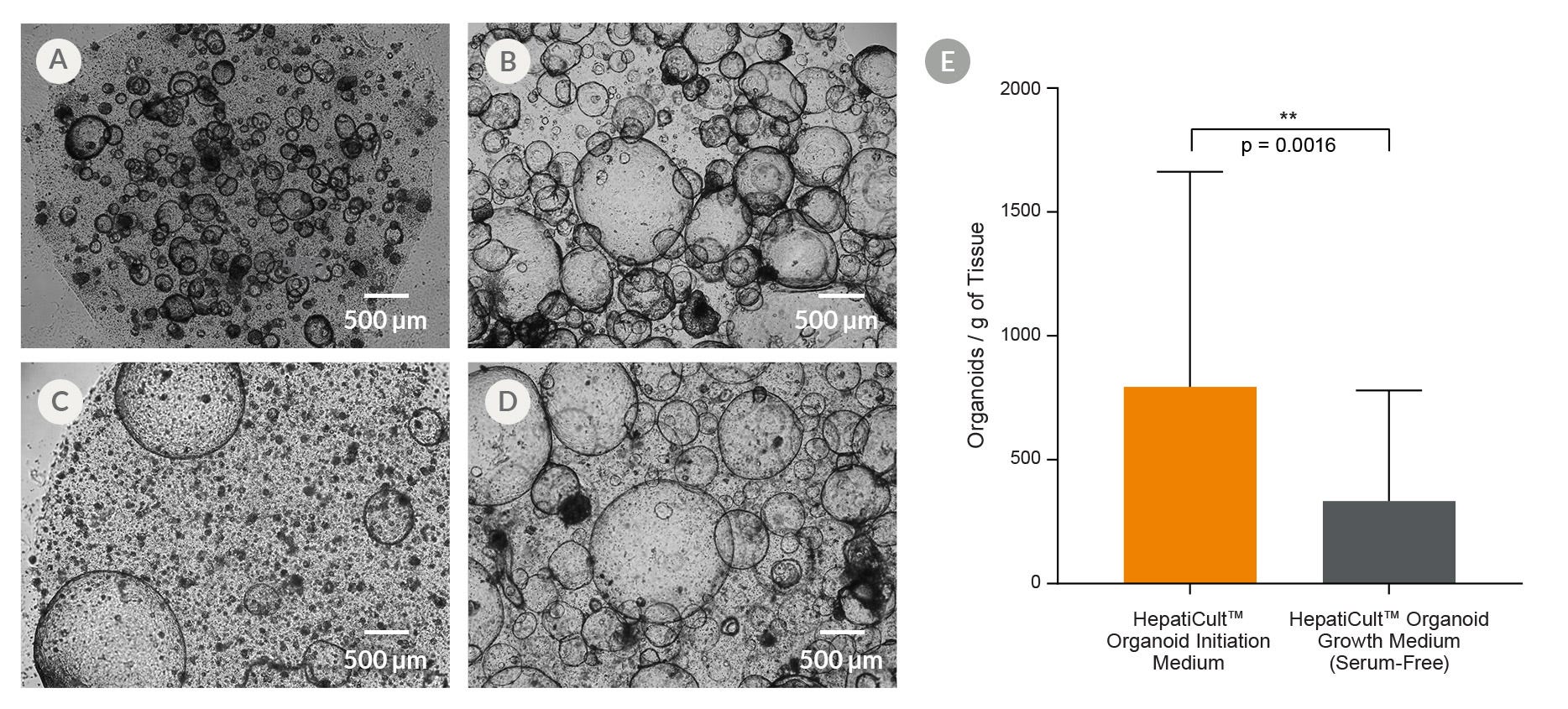
Figure 2. HepatiCult™ Organoid Kit (Human) Provides Efficient Organoid Initiation From Human Liver Tissue
(A) Organoid cultures were initiated in HepatiCult™ OIM, and then (B) passaged into HepatiCult™ OGM. For serum-free culture conditions, organoid cultures were both, (C) initiated in and (D) passaged in HepatiCult™ OGM. Culture images shown are from (A, C) day 15 following initiation, and (B, D) on day 8 of the first passage. (E) Quantification of organoid initiation efficiency shows a significantly higher organoid yield in HepatiCult™ OIM per gram of human liver tissue (mean ± SD; n=14).
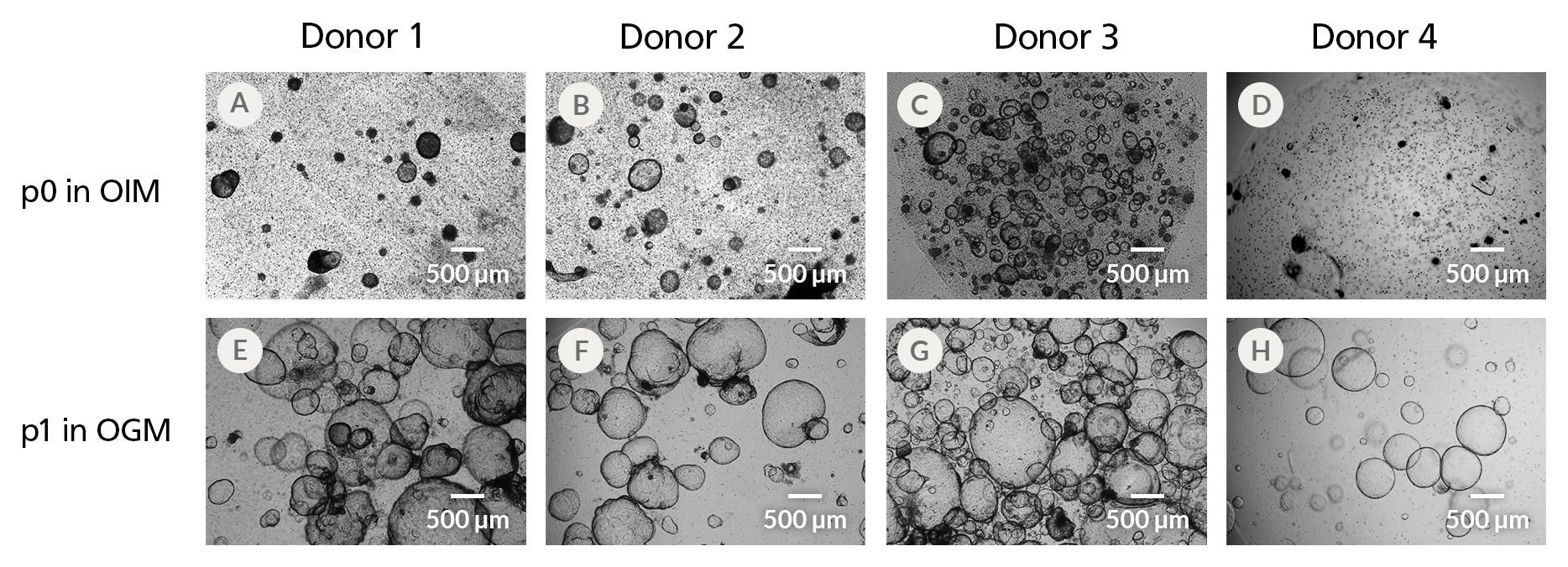
Figure 3. HepatiCult™ Organoid Initiation Medium (Human) Supports Robust Organoid Establishment Across Multiple Liver Tissue Donor Samples
Organoids initiated from 4 donor tissue samples (A-D) exhibit morphological heterogeneity 15 days after initiation. All initiated cultures were subsequently expanded in HepatiCult™ OGM using a 1:1 passaging ratio (E-H), yielding healthy organoids at the end of the first passage.
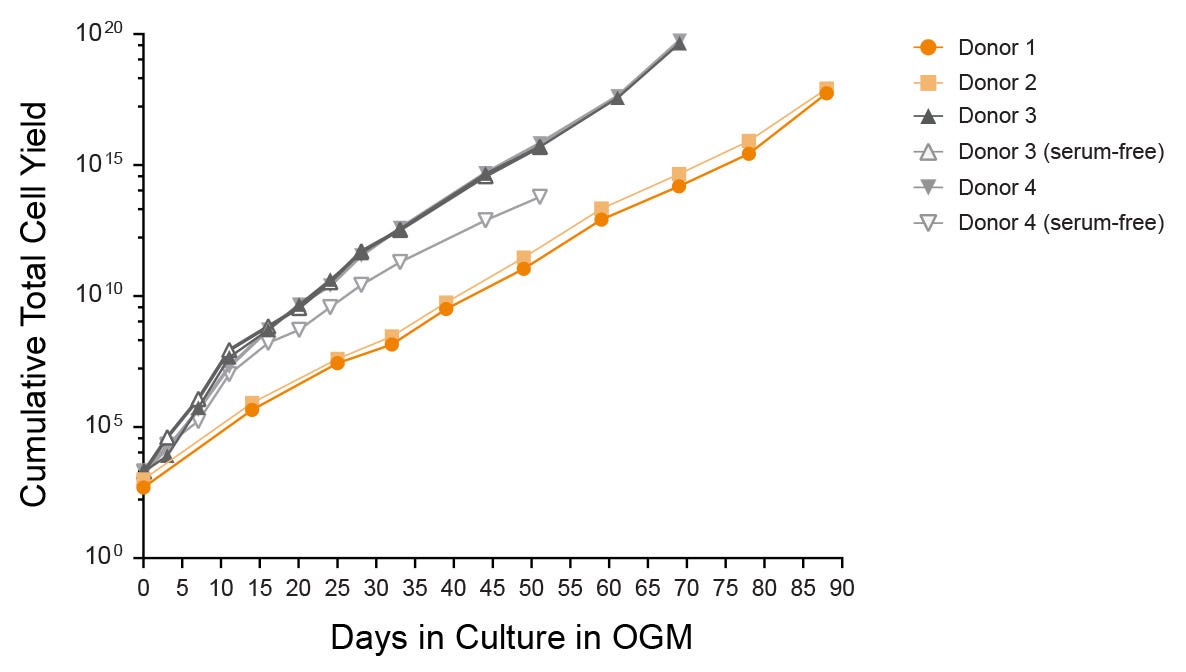
Figure 4. Expansion of Organoid Cultures in HepatiCult™ Organoid Growth Medium
Hepatic organoids show efficient growth in HepatiCult™ OGM across multiple donors and passages with potential for indefinite culture. Organoids initiated using the serum-free workflow exhibited comparable growth rates when expanded in HepatiCult™ OGM (open markers).
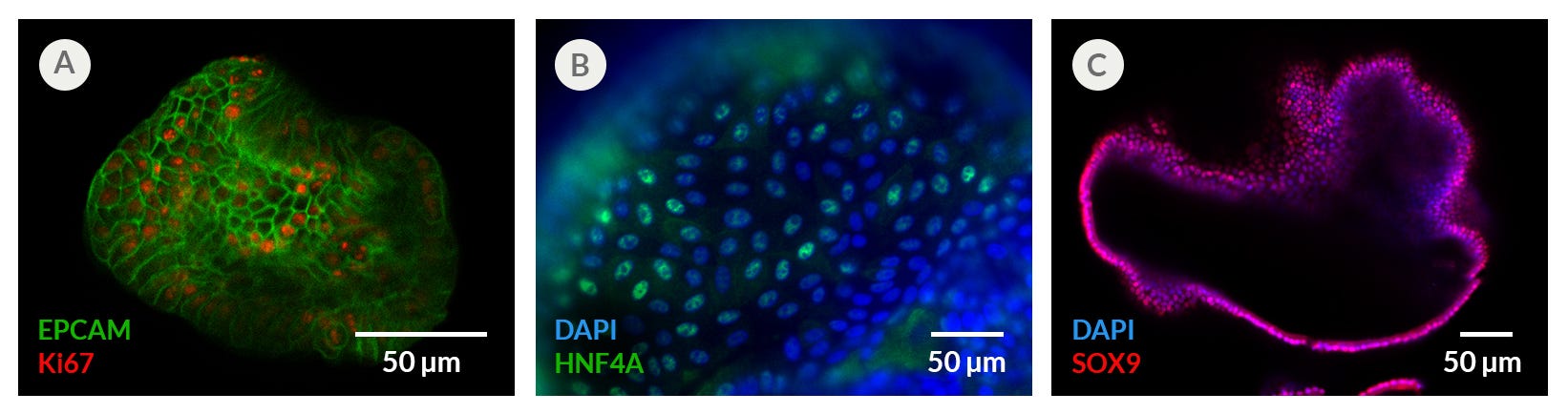
Figure 5. Proliferating Hepatic Organoids Display Characteristics of Hepatic Progenitors
Human liver organoids grown in HepatiCult™ OGM display characteristics of proliferating hepatic progenitors observed through immunocytochemistry staining of (A) KI67, (B) HNF4A and (C) SOX9. Proliferating hepatic organoids also display characteristics of the hepatic epithelium including expression of (A) EPCAM. (B, C) Nuclei are counterstained with DAPI.
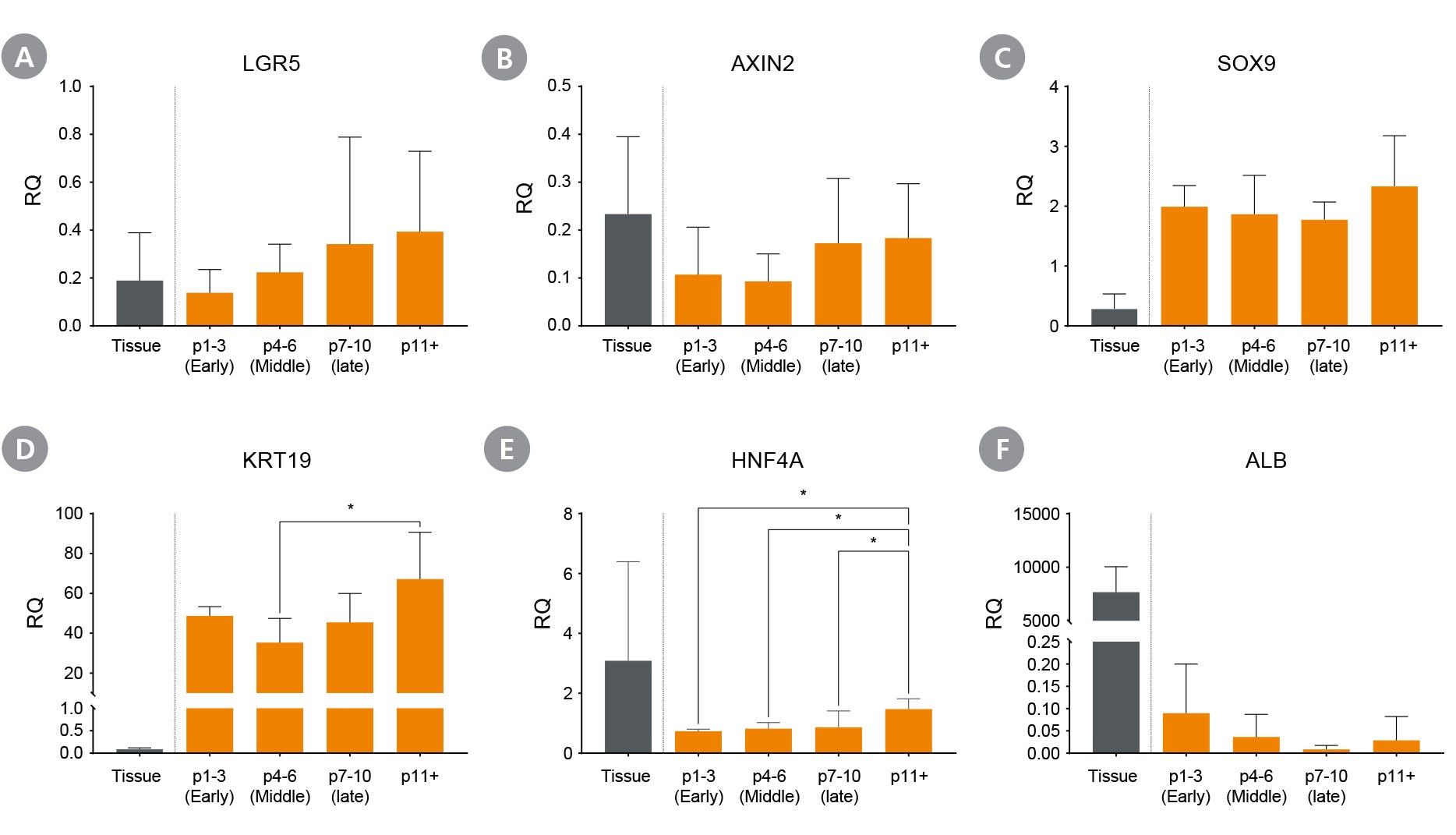
Figure 6. Proliferating Hepatic Organoids Maintain Genetic Expression Across Multiple Passages
Liver organoids maintained in HepatiCult™ OGM express stem cell markers (A) LGR5 and (B) AXIN2, ductal markers (C) SOX9 and (D) KRT19, as well as hepatic marker (E) HNF4a and (F) Albumin (ALB) across multiple passages, with minimal albumin expression observed during culture in HepatiCult™ OGM. Expression levels were measured by qPCR and normalized to TBP and UBC housekeeping genes to quantify relative expression levels. (mean ± SD; n = 2-5 organoid lines), * p < 0.05.
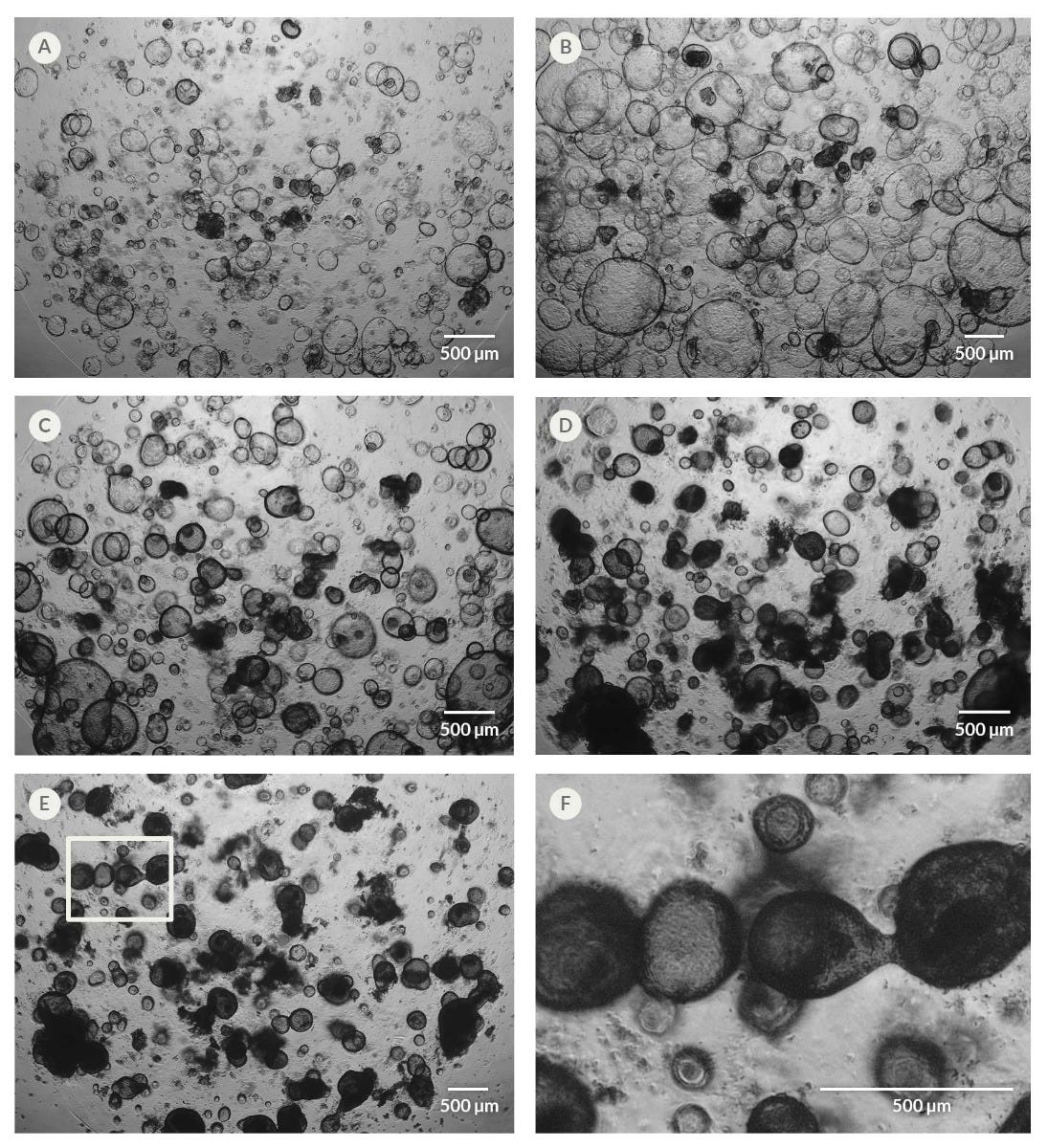
Figure 7. Organoid Differentiation Induces Changes in Organoid Morphology
Organoids exhibit a compact and dense morphology, often comprising thickened epithelia, upon switching cultures to HepatiCult™ Organoid Differentiation Medium (ODM). Shown are images of the same culture well over the course of the differentiation, including (A) day two of culture in HepatiCult™ OGM, (B) day five of culture, immediately after switching organoid cultures from HepatiCult™ OGM to HepatiCult™ ODM, (C) day seven of culture (two days after switching to ODM), (D) day ten of culture (five days after switching to ODM), and (E) day 15 of culture (ten days after switching to ODM). (F) Magnification of the rectangular section highlighted in (E).

Figure 8. Differentiation of Hepatic Organoids in HepatiCult™ ODM Induces Changes in Gene Expression Consistent with Hepatic Maturation As Analysed by RNA Sequencing
Organoids expanded in HepatiCult™ OGM express markers associated with proliferative hepatic progenitor and ductal cells. Upon differentiation in HepatiCult™ ODM, organoids shift towards a more mature state, with increased expression levels of genes associated with key hepatic functions. This includes serum protein synthesis (ALB, SERPINA1), phase 1 and 2 drug metabolism (CYP2C9, CYP3A4, UGT1A1, GSTA1), glucose metabolism (SLC2A2, G6PC), bile acid synthesis (CYP27A1, BAAT), and urea synthesis (OTC). Genes associated with lipid and cholesterol metabolism are expressed at similar levels in both OGM and ODM organoids. Columns represent 3 biological replicates analyzed by bulk RNA-seq at passages 7 - 9 on day 5 in HepatiCult™ OGM and day 15 in HepatiCult™ ODM. The housekeeping gene, TBP, is included as a reference.
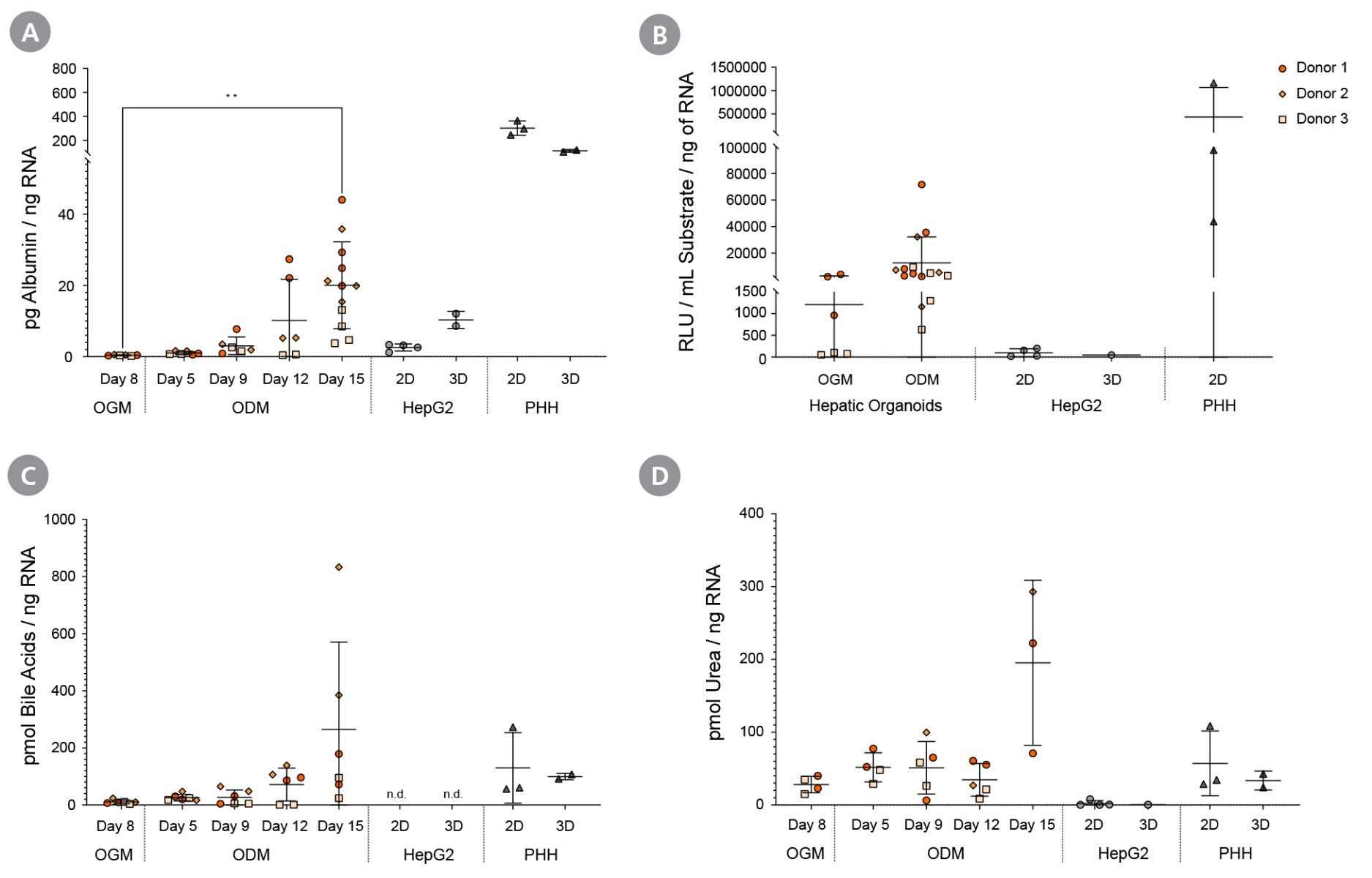
Figure 9. Differentiated Hepatic Organoids Demonstrate Functionality of Mature Hepatocytes
Upon differentiation in HepatiCult™ ODM, liver organoids were assayed for (A) albumin secretion, (B) CYP3A4 activity, (C) total bile acid production, and (D) urea production. Hepatic functionalities were compared to HepG2 cells and primary human hepatocytes (PHH), which were cultured in supplier-recommended media. Albumin secretion was detected using an ELISA kit (Abcam), total bile acid and urea production were analysed using colorimetric kits (Abcam), and CYP43A4 activity, referring to baseline activity without induction, was determined using the Luciferin-IPA kit (Promega). (mean ± SD; n = 3 organoid lines across 2 experiments, n = 2-3 technical replicates of HepG2 in 1 experiment, and n = 3 cryopreserved PHH donor samples in 1 experiment), * p < 0.05; ** p < 0.01.

Figure 10. CYP3A4 Activity Can Be Modulated in Differentiated Hepatic Organoids
Organoid cultures in HepatiCult™ ODM from three different donors were assessed for CYP3A4 (A) activity through LC-MS/MS and (B) gene expression through qPC R, demonstrating a time-dependent increase for both measurements. Gene expression levels were normalized to housekeeping genes, TBP and UBC, and ‘Day 0’ expression. To evaluate if CYP3A4 activity could be modulated, two differentiated hepatic organoid lines were treated with (C) calcitriol or (D) ketoconazole. CYP3A4 activity was assessed on day 15 of differentiation, 24 (open markers), or 48 hours post-treatment using the P450-Glo™ CYP3A4 assay (Luciferin-IPA) (Promega). Both differentiated hepatic organoid lines demonstrated a significant increase in CYP3A4 activity with increasing concentrations of calcitriol and a significant decrease in CYP3A4 activity with increasing concentrations of ketoconazole (n = 2 organoid lines, mean values from 2 experiments). Error bars = SD. One-way ANOVA with Dunnett's multiple comparisons test was used for statistical testing (**P ≤ 0.01, ****P ≤ 0.0001, ns = not significant).

Figure 11. High Drug Concentrations Disrupt Albumin Secretion in Differentiated Hepatic Organoids
Organoids from two different donors were differentiated in HepatiCult™ ODM and treated with (A) troglitazone, (B) ketoconazole, (C) rifampicin, or (D) acetaminophen on day 7 of differentiation when the last media refresh was performed. Spent media was collected three days later on day 10, and accumulated albumin was measured using the MSD R-PLEX Human Albumin Assay. Organoids were also collected on day 10 and lysed for (E) viability assessments using the CellTiter-Glo® 3D Cell Viability Assay (Promega). Both differentiated hepatic organoid lines demonstrated a significant drop in secreted albumin upon treatment with high drug concentrations, corresponding with a decrease in cell viability (mean values from 2 experiments). Error bars = SD. One-way ANOVA with Tukey multiple comparisons test used for statistical testing (single asterisk or hash indicate P < 0.05, double asterisk or hash indicate P ≤ 0.01, triple asterisk or hash indicate P ≤ 0.001, quadruple asterisk indicate P ≤ 0.0001).

Figure 12. Primary Hepatic Organoids Are Sensitive to Drug-Induced Hepatotoxicity in a Dose-Dependent Manner
Proliferating and differentiated hepatic organoids were treated with (A) troglitazone, (B) ketoconazole, (C) rifampicin, (D) acetaminophen, or 1% DMSO vehicle control for 3 days, with full-medium changes containing fresh compounds every 24 hours. Cell viability was assessed 24 hours after the last compound addition using the CellTiter-Glo® 3D Cell Viability Assay (Promega), and IC50 values were determined. These drug dose-response viability curves were compared to similarly treated HepG2 cells and primary human hepatocytes (PHH) cultured in 2D. Error bars = SD.

Figure 13. HepatiCult™ Organoid Kit Supports the Growth and Differentiation of Porcine Liver Organoids
Porcine liver organoids were established in HepatiCult™ Organoid Initiation Medium, and subsequently expanded for 4 passages in (A) HepatiCult™ Organoid Growth Medium (Human) and differentiated in (B, C) HepatiCult™ Organoid Differentiation Medium (Human). Organoids are stained with basolateral protein marker p120 (green), apical membrane and bile canaliculi marker F-Actin (purple), and nuclear dye Hoechst (blue). (D) Magnification of Panel C indicates bile canaliculi formation as seen through F-Actin staining. Scale bars = 50 μm. Data used with permission from Dr. Amy Engevik (Vanderbilt University Medical Center)
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Resources and Publications
Educational Materials (23)
Related Products
-
 HepatiCult™ Organoid Growth Medium (Mouse)
HepatiCult™ Organoid Growth Medium (Mouse)Cell culture medium for establishment and maintenance of mouse hepatic progenitor organoids
-
 CryoStor® CS10
CryoStor® CS10Animal component-free, defined cryopreservation medium with 10% DMSO
-
 STEMdiff™ Hepatocyte Kit
STEMdiff™ Hepatocyte KitSerum-free differentiation kit for the generation of hepatocyte-like cells from human PSCs
-
 Mouse Hepatic Organoids
Mouse Hepatic OrganoidsCryopreserved mouse hepatic progenitor organoids for establishment of organoid cultures
Item added to your cart

HepatiCult™ Organoid Kit (Human)
This product was developed under a license to intellectual property owned by Hubrecht Organoid Technology (HUB). This product is sold for research use only. Purchase of this product does not include the right touse it for drug screening aiming for commercial gain, equipment validation, biobanking, or for other commercial purposes. Purchasers wishing to use the product for purposes other than basic research use shouldcontact HUB at www.huborganoids.nl to obtain a further license. Purchasers may apply for a License from HUB, which will not be unreasonably withheld by HUB.
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.























