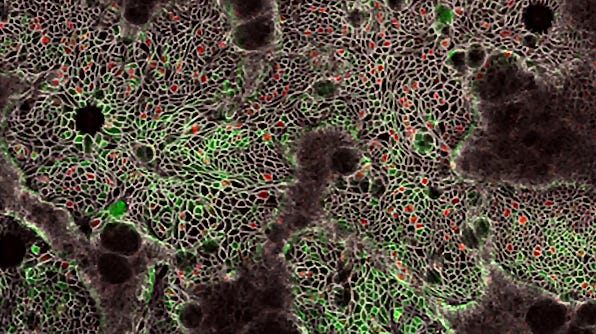
Establishment of 3D Organoid-Derived Hepatic Monolayers Using HepatiCult™ Organoid Initiation Medium (Human)
Growing hepatic organoids as organoid-derived monolayers offers further flexibility for experimental design and decreased interference from the extracellular matrix. Organoid-derived monolayers can additionally be grown on Transwell® inserts, enabling different treatments in each of the compartments.
The below protocol is for the establishment of hepatic organoid-derived monolayers using HepatiCult™ Organoid Initiation Medium (Human).
Materials
- HepatiCult™ Organoid Initiation Medium (Human) (Catalog #100-0384)
- Healthy hepatic organoid cultures expanded in HepatiCult™ Organoid Growth Medium (Human)
- DMEM/F-12 + 15 mM HEPES (Catalog #36254)
- 25% Bovine serum albumin (BSA) solution in water
- Corning® Matrigel® (Corning 356231, ≥ 8mg/mL protein)
- Sterile phosphate-buffered saline (PBS)
- Antibiotics (e.g. gentamicin)
- TrypLE™ Express Enzyme (1x), no phenol red (Thermo Fisher 12605010)
- 5 mM Y-27632 (ROCKi; Catalog #72302) solution in water
- Anti-Adherence Rinsing Solution (Catalog #07010)
- 15 mL and 50 mL conical tubes (Catalog #38009 and Catalog #38010)
- Costar® 6.5 mm Transwell®, 0.4 µm Pore Polyester Membrane Inserts (recommended) (Catalog #38024) OR 24-well tissue culture-treated plate (Catalog #38017)
- Sterile 200 µL and 1000 µL tips
- 10 mL serological pipettes (Catalog #38004)
- Styrofoam box with ice
- Water bath at 37°C
- Incubator with atmospheric O2 and 5% CO2 at 37°C
Preparation
- Thaw HepatiCult™ Organoid Growth Supplement and Organoid Supplement overnight at 2 - 8°C. Prepare 100 mL of complete HepatiCult™ Organoid Initiation Medium using sterile technique:
- 5 mL HepatiCult™ Organoid Growth Supplement
- 50 mL Organoid Supplement
- 44.8 mL HepatiCult™ Organoid Basal Medium
- 200 µL of 5 mM Y-27632 (final concentration 10 µM)
- Add antibiotics (e.g. 50 µg/mL gentamicin)
- Mix thoroughly. Warm complete HepatiCult™ OIM to room temperature (15 - 25°C) before use.
Note: Once thawed, use HepatiCult™ Organoid Growth Supplement and Organoid Supplement immediately or aliquot and store at -20°C. After thawing aliquots, use immediately. Do not re-freeze.
Note: If not used immediately, store complete HepatiCult™ OIM at 2 - 8°C for up to 2 weeks.
- Prepare 50 mL serum-supplemented DMEM/F-12 + 15 mM HEPES (DMEM + BSA):
- 2 mL 25% BSA solution in water (1% final concentration)
- 100 µL 5 mM Y-27632 (final concentration 10 µM)
- 47.9 mL DMEM/F-12 + 15 mM HEPES
- Mix thoroughly. Store on ice.
- Thaw Matrigel® on ice. Prepare the coating solution by combining thawed Matrigel® with ice-cold PBS at a 1:50 dilution (i.e. 2% solution). Coating volumes required are:
- 100 µL per well for Transwell® plates
- 200 µL per well for 24-well plates
- Coat culture plate(s) with a dilute Matrigel® solution:
- Add an appropriate volume of coating solution to each well to be used for monolayer generation. Ensure the entire well surface is evenly coated.
- Incubate at 37°C for at least 1 hour before use. Do not let the Matrigel® solution in the wells evaporate.
- Prepare 10 mL of TrypLE™ + BSA:
- 400 µL 25% BSA solution in water (1% final concentration)
- 10 mL TrypLE™ Express Enzyme
- Mix thoroughly. Store on ice.
- Pre-wet all serological pipettes, pipette tips, and conical tubes that will come in contact with the organoid-derived cells, first with Anti-Adherence Rinsing Solution and then with DMEM/F-12 + 15mM HEPES.
Protocol
- Check that the Matrigel® domes to be passaged are intact (i.e. the whole dome remains attached to the plate and no loose Matrigel® pieces or organoids are observed in the well). If the dome is intact, proceed to step 2. If the dome is loose, remove at least 500 µL medium using a 1 mL pipettor, and add 1 mL TrypLE™ + BSA to the well to 1mL; proceed to step 4.
- Without touching the dome, aspirate and discard the medium in each well to be passaged.
- Using a 1 mL pipettor, forcefully add 1mL of TrypLE™ + BSA to the center of each dome, then let sit for 1 minute.
- Using a 1 mL pipettor, vigorously pipette the total volume in the well up and down 45 times, taking care not to generate bubbles.
Note: This results in mechanical breakdown of organoids and Matrigel® into a single-cell suspension. Some smaller fragments may remain that can be further dissociated by incubating the suspensions at 37°C for 5 - 10 minutes with periodic trituration/agitation using a pipette or vortex.
- Add 1 mL cold DMEM + BSA to each well. Transfer the entire volume of single-cell suspensions from each well to a 15 mL conical tube on ice.
Note: Pool the contents of up to 4 wells in one 15 mL tube.
- Wash the wells that the organoids were harvested from with 1 mL cold DMEM + BSA. Add this volume to the 15 mL tube prepared in step 5.
- Add ice-cold DMEM + BSA to each tube so that the total volume of each tube is 12 mL. Centrifuge tube(s) at 290 x g for 5 minutes. Aspirate as much of the supernatant as possible without disturbing the pellet.
- Resuspend cells in 2 mL of DMEM + BSA.
Note: If more than 4 wells were dissociated, cells from all processed wells can be pooled into the same 15 mL tube at this stage.
- Perform a live cell count and determine the number of wells to be seeded for monolayer generation. The recommended seeding densities are as follows:
- 1.0 x 105 cells per Transwell®
- 3.0 x 105 cells per well of a 24-well plate
Note: These densities will typically yield 100% confluent hepatic monolayers within 2 days. Alternative seeding densities can be used if confluent monolayers are desired at a different timepoint.
- Centrifuge tube(s) at 290 x g for 5 minutes. Aspirate as much of the supernatant as possible without disturbing the pellet.
- Resuspend cells in complete HepatiCult™ OIM to generate a single-cell suspension. Place tube on ice.
Note: If using the recommended seeding densities in step 9, resuspend cells at 2.0 x 106 live cells/mL.
- Remove the coated plate from the incubator and place in the biosafety cabinet.
- Gently tilt the plate. Aspirate and discard the excess Matrigel® volume that collects at the edge of the well, ensuring that the coated surface is not scratched.
- Immediately add complete HepatiCult™ OIM to each well to be seeded, as follows:
- 100 µL in the upper chamber and 500 µL in the lower chamber per Transwell®
- 350 µL per well of a 24-well plate
- Slowly add the single-cell suspension generated in step 11 to each well, as follows:
- 50 µL of suspension per Transwell® (i.e. 1.5 x 105 cells/Transwell® if using a 2.0 x 106 live cells/mL suspension)
- 150 µL of suspension per well of a 24-well plate (i.e. 4.5 x 105 cells/well if using a 2.0 x106 live cells/mL suspension)
Note: Monolayers typically achieve 100% confluence within 2 - 3 days if seeded at the indicated density.
- Incubate the plate at 37°C and 5% CO2. Monitor growth daily.
- Perform a full-medium change every 2 - 3 days by carefully aspirating the medium and adding 750 µL of fresh complete HepatiCult™ OIM at room temperature (15 - 25°C).
Note: To avoid weekend medium changes, perform medium changes on Mondays, Wednesdays, and Fridays.
Note: Monolayers can be used in assays once they are confluent. Once established, monolayers remain viable for at least 10 days. Multi-layering of cells may be observed if confluent monolayers are maintained for longer periods.
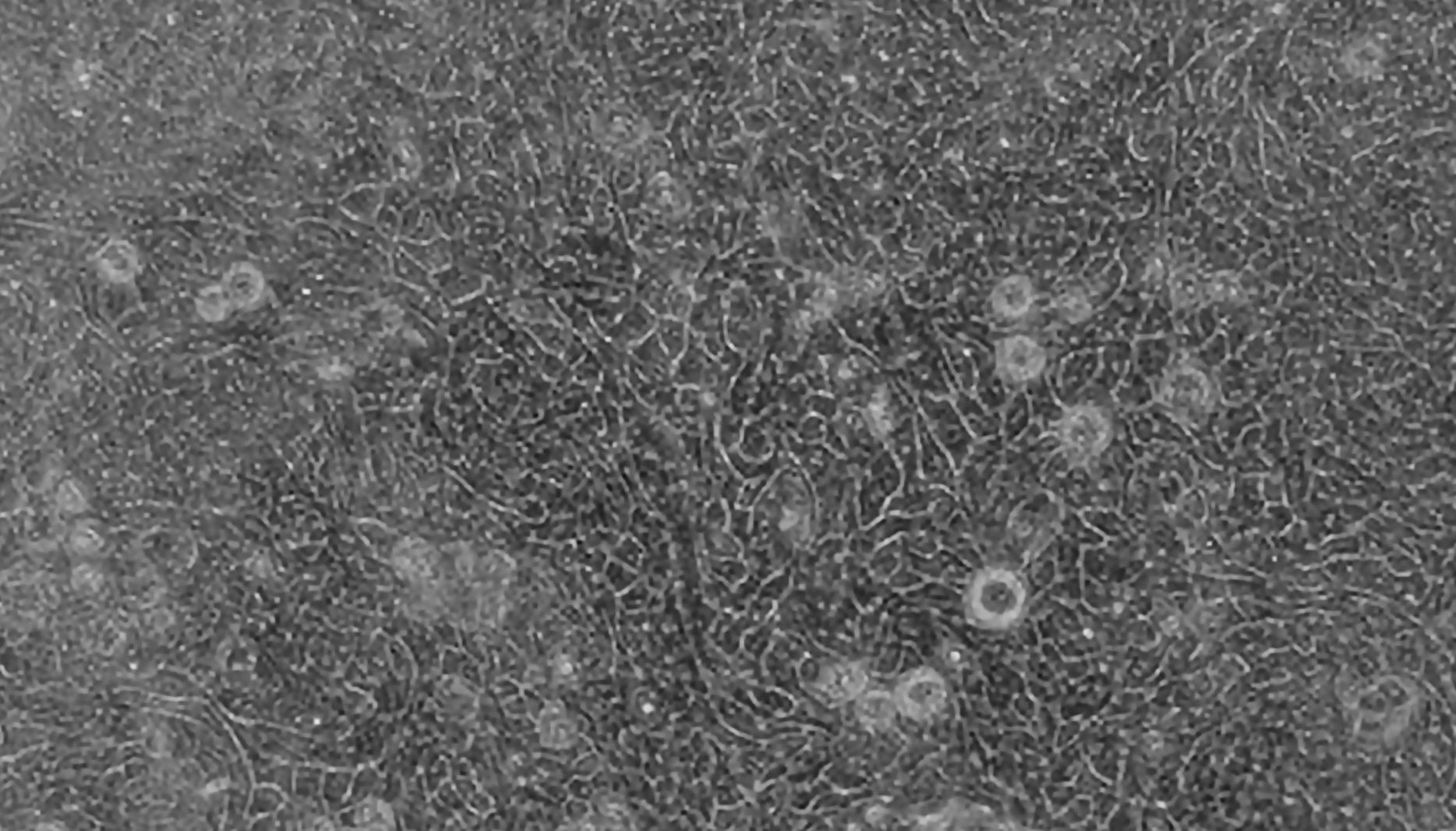
Figure 1. A 3D-Organoid-Derived Hepatic Monolayer on a Transwell® Insert
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration