How to Perform Immunocytochemistry (ICC) Staining of Epithelial Cells Cultured as Monolayers or at the Air-Liquid Interface
How to perform immunocytochemistry (ICC) staining of air-liquid interface or monolayer cultures in your own lab
Air-liquid interface (ALI) or monolayer cultures of human epithelial cells are increasingly being adopted as a two-dimensional (2D) model system to study human health and disease in vitro. By removing culture medium from the apical surface of the cells, airway and gastrointestinal cultures undergo further differentiation that provides a more physiological representation of the in vivo conditions. ALI cultures grown using PneumaCult™-ALI Medium (Catalog #05001) or PneumaCult™-ALI-S Medium (Catalog # 05050) express characteristic cellular markers for the large airway (e.g. tight junctions and ciliated cells) and small airway (e.g. club cells and secretory proteins) respectively. Similarly, intestinal monolayers and intestinal ALI cultures differentiated using IntestiCult™ Organoid Differentiation Medium (Human) (Catalog # 100-0214) express higher levels of key differentiation markers (e.g. goblet cells, enterocytes, and enteroendocrine cells).
Using immunocytochemistry (ICC) staining, a laboratory technique to visually confirm the expression and localization of cellular markers in cell cultures, researchers can detect relevant epithelial markers and check for successful ALI and monolayer culture differentiation. Besides end-point characterization studies, ICC staining also enables researchers to evaluate different experimental conditions. For instance, it can be used to assess marker expression in the culture in the presence or absence of a specific drug or pathogens. ICC staining has also been used to show expression of angiotensin-converting enzyme 2 (ACE2), the SARS-CoV-2 viral receptor protein, on ALI (Figure 2) and monolayer cultures of different epithelial tissues.
Below, we describe a protocol for whole-mount ICC staining of ALI cultures derived from any tissue type or monolayer cultures (derived from primary cells, pluripotent stem cells, or cell lines) grown in Transwell® inserts.
Materials
- Normal serum (use the same species as used to generate the secondary antibody)
- Bovine serum albumin (BSA)
- Cold fish skin gelatin
- Triton™ X-100
- TWEEN® 20
- Sodium azide (optional)
- Phosphate-buffered solution (PBS; Catalog #37350)
- DAPI (Catalog #75004)
Although this immunocytochemistry staining procedure can be performed at any point during the ALI culture differentiation phase, the highest degree of differentiated cell staining and visualization will take place after full ALI culture maturity. For information related to differentiation of human bronchial epithelial cells (HBECs), human small airway epithelial cells (HSAECs) or human intestinal cells, refer to the Product Information Sheet (PIS) for PneumaCult™-ALI Medium, PneumaCult™-ALI-S Medium or IntestiCult™ Organoid Differentiation Medium (Human), respectively.
Protocol
I. Buffer Preparation
- Prepare 100 mL of blocking buffer by combining the components in the order listed in Table 1. Mix the solution thoroughly after adding each component.
- Store the blocking buffer at 2 - 8°C.
*The Normal serum must be of the same species as used to generate the secondary antibody.ComponentQuantityFinal concentration in solution after additionPBS97.85 mL--Normal serum*2 mL2 %BSA1 g1 %Cold fish skin gelatin100 mg0.1 %Triton™ X-100100 μL0.1 %TWEEN® 2050 μL0.05 %Sodium azide**50 mg0.05 %
** Sodium azide is an antibacterial agent added to prevent bacterial growth in the buffer when stored for longer periods. It is very toxic and is dispensable as long as the buffer is used quickly (within a few days). - Prepare 100 mL of primary antibody dilution buffer by combining components in the order listed in Table 2. Mix the solution thoroughly after adding each component.
- Store the primary antibody dilution buffer at 2 - 8°C.
ComponentsQuantityFinal concentration in solution after additionPBS100 mL--BSA1 g1 %Cold fish skin gelatin100 mg0.1 %Sodium azide50 mg0.05 % - Prepare 100 mL of secondary antibody dilution buffer by combining components in the order listed in Table 3. Mix thoroughly.
Table 3. Preparation of Secondary Antibody Dilution Buffer
ComponentQuantityPBS100 mLSodium azide50 mg- Store the secondary antibody dilution buffer at 2 - 8°C.
- Prepare 1 mL of DAPI staining stock solution by combining components in the order listed in Table 4. Mix thoroughly and aliquot the solution into volumes of 50 μL.
Table 4. Preparation of DAPI Staining Stock Solution
ComponentQuantityPBS1 mLDAPI5 mg- The DAPI staining stock solution will have a final concentration of 5 mg/mL.
- Store the DAPI staining stock solution at -20°C.
- Retrieve the ALI cultures growing in Transwell® inserts in a cell culture plate from the incubator.
- In a biosafety cabinet, remove the culture medium from the plate wells, and wash the ALI cultures 5X with room temperature PBS for 5 minutes each. Carefully aspirate PBS, making sure not to touch the culture.
Note: If your ALI cultures are undergoing electrophysiology analysis in an Ussing chamber, retrieve them, remove any excess buffer from the Transwell® inserts, and proceed to the next step. - Place the plate containing Transwell® inserts in ice-cold (-20°C) methanol and seal the plate with Parafilm®. Incubate the plate overnight at -20°C.
- Remove the methanol from the ALI cultures by quickly inverting each Transwell® insert, and dabbing on a Kimwipe™.
- Dip the bottom portion of the inserts, containing the polyester membrane, into -20°C cold acetone for 1 minute. After 1 minute, dab on a Kimwipe™ to remove any excess acetone, and set upside down to air-dry. Once dry, store the plate containing Transwell® inserts at 2 - 8°C until needed, or proceed to Step III: Blocking.
- Gently wipe the bottom of each Transwell® insert with a PBS-soaked Kimwipe™.
- Wash each insert 3X with PBS for 5 minutes each, adding 200 μL PBS to the apical compartment and 500 μL PBS to the basal compartment. After each wash, use an aspirator to carefully remove the PBS.
- Incubate the inserts with the blocking buffer (prepared in section I) at room temperature for 1 hour.
Note: Rocking of the plates is not necessary, but beneficial when possible. - Before proceeding with primary antibody addition, wash each insert 3X with PBS for 5 minutes each.
- Add primary antibodies of your choice to the primary antibody buffer at the desired dilution. Add 200 μL of this diluted mixture to the apical compartment and 500 μL to the basal compartment.
- Incubate overnight at 2 - 8°C.
Note: Rocking of the plates is not necessary, but beneficial when possible. - Remove the primary antibody buffer from each insert by aspiration, and then wash them again 3X with PBS, for 5 minutes each.
- Add relevant secondary antibodies into the secondary antibody buffer at a dilution of 1:1000. Add 200 μL of this diluted mixture to the apical compartment and 500 μL to the basal compartment.
- Incubate the inserts at room temperature for 2 hours in the dark.
Note: After adding the conjugated secondary antibody, avoid exposing the plate to light to prevent any fluorescence quenching.Table 5. Recommended Antibodies for Staining Airway Epithelial Cells
ProductCatalog #Culture TypeAnti-Mucin 5AC Antibodye.g. Abcam, ab212636Large airway ALI culturesCC10 Antibody (SCGB1A1)e.g. Santa Cruz Biotechnology, sc-365992Small airway ALI culturesAnti-SCGB3A2 Antibodye.g. Abcam, ab181853Small airway ALI cultures
Table 6. Recommended Antibodies for Staining Intestinal Monolayers
- Wash the inserts 3X with PBS, for 5 minutes each.
- Add DAPI staining stock solution into PBS at a dilution of 1:1000. Add 200 μL of this diluted mixture to the apical compartment and 500 μL to the basal compartment.
- Incubate at 2 - 8°C for 10 - 15 minutes.
- Wash each insert once with PBS for 5 minutes.
- Aspirate the PBS from the individual inserts, then peel or cut the polyester membrane from the Transwell® hanger (Figure 1). Once separated from the hanger, use surgical scissors to cut away the curved membrane edge. This will allow the stained culture membrane to sit flat under the coverslip during the mounting procedure.
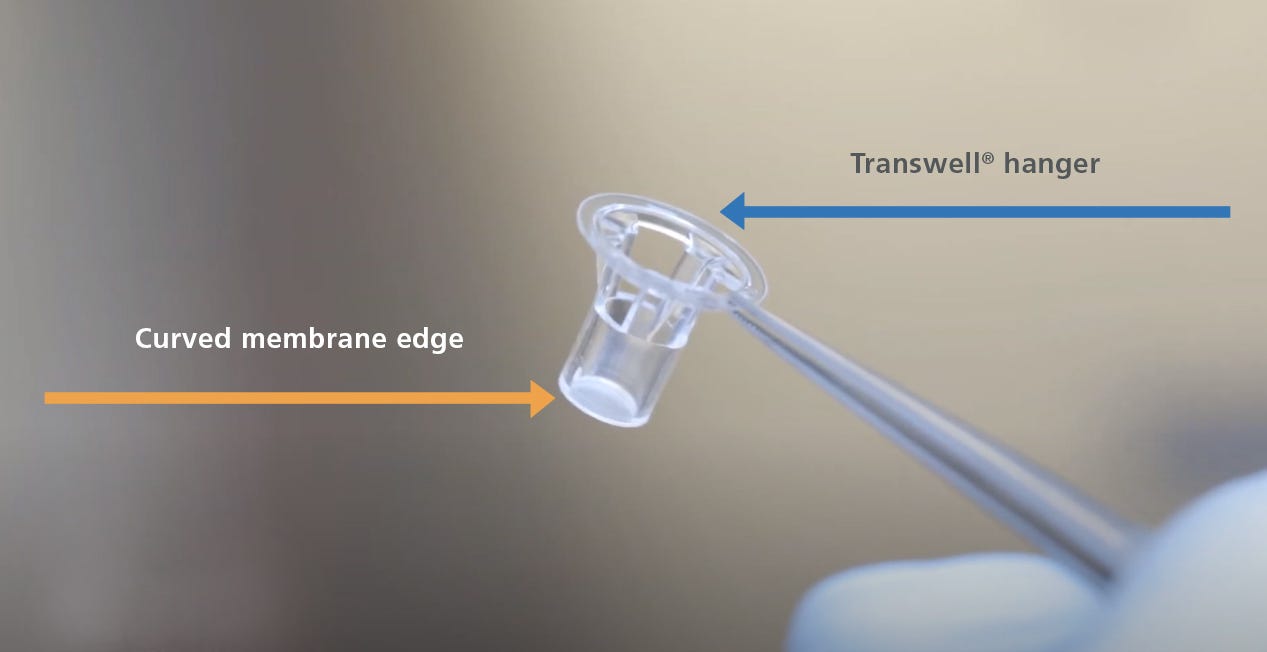
Figure 1. The Hanger and Curved Membrane Edge of a Transwell® Insert
- Using a plastic Pasteur pipette, add one droplet of an anti-fade solution or liquid mountant to a microscope slide for every stained culture membrane to be mounted.
Note:- Do not exceed three culture membranes to be mounted on a single slide.
- To prevent air bubbles being caught under the coverslip from step 24, add a small volume of liquid mountant in a line to connect the existing droplets of the solution.
- Using forceps, place one stained culture membrane, cell-side up, on a droplet of liquid mountant. Add extra liquid mountant on top of the membrane, so as to completely cover the membrane’s surface. Carefully place a coverslip on top of the membranes covered in liquid mountant.
- Allow the slides to cure at room temperature overnight in the dark, sitting horizontally flat.
- On the following day, visualize and take images of the stained membranes using a confocal microscope at 63X oil immersion.
II. Plate Preparation
III. Blocking
IV. Primary Antibody Addition
V. Secondary Antibody Addition
VI. Mounting and Visualization
Data
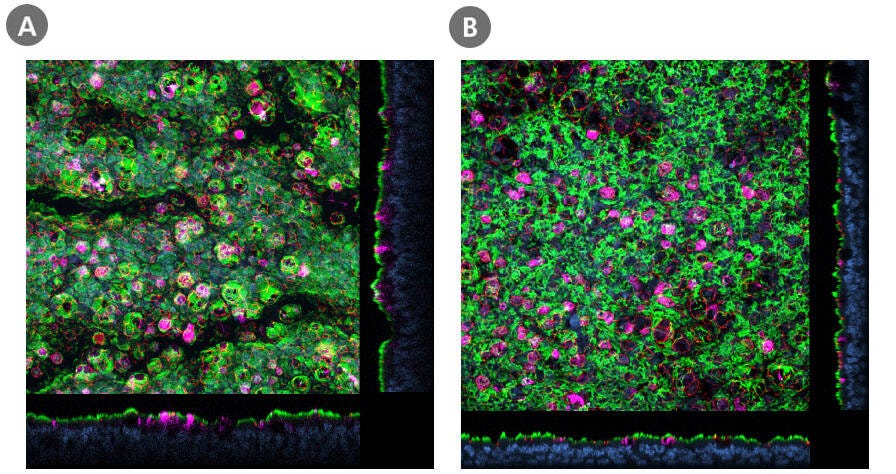
Figure 2. Air-Liquid Interface Cultures of HBECs and HSAECs Express ACE2
Confocal images of whole mount immunostained ALI cultures of (A) HBECs cultured in PneumaCult™-ALI Medium and (B) HSAECs cultured in PneumaCult™-ALI-S Medium, at P2. The ALI cultures were fixed and stained with antibodies for ciliated cells (AC-tubulin; green), SARS-CoV-2 receptor (ACE2; magenta), and tight junctions (ZO1; red). The nuclei were counterstained with DAPI (blue). ACE2 was detected in both (A) large and (B) small airway culture systems during early passages (≤P6, HBECs; ≤P4, HSAECs). Both HBECs and HSAECs were expanded in PneumaCult™-Ex Plus Medium prior to differentiation at the ALI.
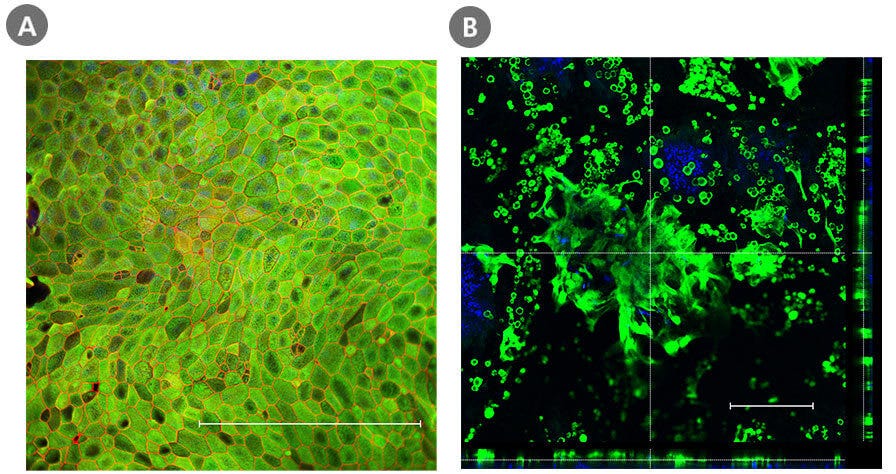
Figure 3. Intestinal Monolayer and ALI Cultures Express Key Differentiation Markers
Organoid-derived monolayers grown as a submerged monolayer (Day 7) or at the ALI (Day 14) using IntestiCult™ Organoid Differentiation Medium (Human) express key differentiation markers. Immunocytochemistry staining of cells in the submerged monolayer show marker expression of enterocytes (KRT20, green) and tight junctions between cells (ZO-1; red), whereas those cultured at the ALI show an increased secretory cell differentiation evidenced by the staining of goblet cells (MUC2; green). The nuclei were counterstained with DAPI (blue). Scale bar = 250 µm
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration




