How to Thaw Intestinal Organoids
This protocol describes how to thaw frozen mouse or human intestinal organoids using IntestiCult™ Intestinal Organoid Media.
Learn how to cryopreserve intestinal organoids using CryoStor® CS10.
Materials
- IntestiCult™ Organoid Growth Medium (Mouse, Catalog #06005 or Human, Catalog #06010)
- Matrigel® Matrix (e.g. Corning Catalog #354277)
- Antibiotics (e.g Gentamicyn or Penicillin/Streptomycin)
- 24-well plate, tissue-culture treated (e.g. Costar® 24-Well Plate, Catalog #38017 or Falcon® 24-Well Plate, Catalog #38021)
- Bovine serum albumin (BSA)
Protocol
- Thaw 120 μL of Matrigel® Matrix on ice and let previously prepared complete IntestiCult™ Organoid Growth Medium warm to room temperature (15 - 25°C). You will need 3.1 mL of complete media for four wells of a 24-well plate. Place a 24-well tissue culture-treated plate in a CO2 incubator at 37°C to warm for 30 minutes.
- Combine 2 mL of 25% BSA stock solution with 48 mL of DMEM/F-12 with 15 mM HEPES in a 50 mL conical tube to generate a DMEM/F-12 washing solution containing 1% BSA. Leave at room temperature (15 - 25°C) for the duration of this protocol. The washing solution can be stored at 2 - 8°C for up to six months.
- To a 15 mL conical tube, add 2 mL of DMEM/F-12 with 1% BSA solution at room temperature (15 - 25°C).
Note: Cells should be transferred to this tube immediately after thawing to avoid significant reduction in viability. - Retrieve and thaw the frozen organoids by placing the cryovial in a 37°C water bath. Thawing is complete when the freezing medium becomes liquid, at which point the organoids will be visible at the bottom of the tube. Thawing at 37°C should take between 2 and 2.5 minutes; over-warming the medium may affect the growth efficacy of the organoids in culture.
- Wipe the outside of the cryovial with 70% ethanol or isopropanol before opening. Add 1 mL of DMEM/F-12 with 1% BSA solution directly to the cryovial using a 1000 μL pipette. Mix the contents of the cryovial by pipetting up and down four times. Immediately transfer the contents of the cryovial to the 15 mL conical tube containing 2 mL of DMEM/F-12 with 1% BSA solution using a pre-wetted 1000 μL pipette tip.
- Wash the cryovial two more times with 1 mL of DMEM/F-12 with 1% BSA solution and transfer to the conical tube. Be sure to wash the entire inner surface area of the cryovial, including the inside of the lid.
- Wash the organoids to remove the freezing medium by centrifuging the organoid suspension at 200 x g for five minutes at 2 - 8°C. Carefully remove and discard the supernatant. Avoid introducing bubbles. If bubbles are present after centrifugation, carefully aspirate to remove the bubbles first, prior to aspirating the body of the supernatant.
- Using a 200 μL pipette tip, resuspend the organoids by adding 100 μL of room temperature (15 - 25°C) complete IntestiCult™ Organoid Growth Medium.
- Add 100 μL of Matrigel® using a 200 μL pipette tip. Mix the suspension by pipetting up and down five to ten times to ensure a consistent density and viscosity throughout the sample. Avoid introducing bubbles.
- Using a pre-wetted 200 μL pipette tip, add 50 μL of the organoid suspension to four wells of the pre-warmed 24-well plate such that it forms a dome in the center of each well. When plating, dispense to the first stop of the pipette to avoid introducing bubbles. Incubate the organoids at 37°C and 5% CO2 for 10 minutes to allow the Matrigel® to solidify.
- Add 750 μL of room temperature (15 - 25°C) complete IntestiCult™ Organoid Growth Medium to each well containing a Matrigel® dome by pipetting the medium gently down the wall of the well. Do not pipette directly onto the domed cultures.
- Add sterile D-PBS to any unused wells. Place the lid on the culture plate and incubate at 37°C and 5% CO2. Perform a full medium change 3 times per week.
- For the best results, passage the previously frozen organoids two times after thawing before beginning experiments. Organoid growth may be slow in the first passage after thawing. Mouse organoids should be ready for passaging between five and seven days of culture after thawing, and five days after each passage (Figure 1). Human organoids should be ready for passaging at 7 - 14 days of culture after thawing, and 7 - 10 days after each passage (Figure 2).
Note: It is not recommended to expose organoids to consecutive freeze-thaw cycles.
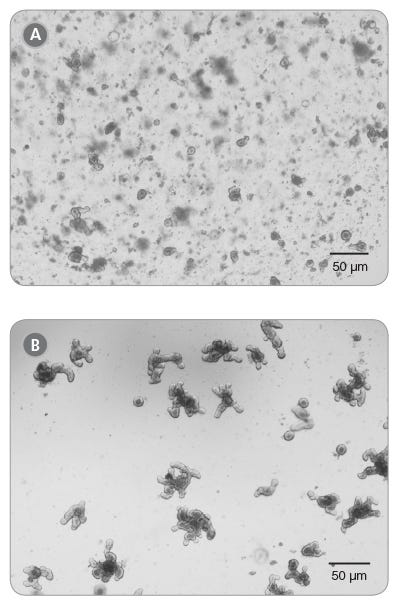
Figure 1. Mouse Intestinal Organoid cultures from Previously Frozen Organoids as Visualized by Light Microscopy
Thawed intestinal organoids were cultured in domes of 1:1 Matrigel® Matrix and IntestiCult™ Organoid Growth Medium and incubated at 37°C and 5% CO2. (A) Passage 0, Day 5; (B) Passage 1, Day 6.
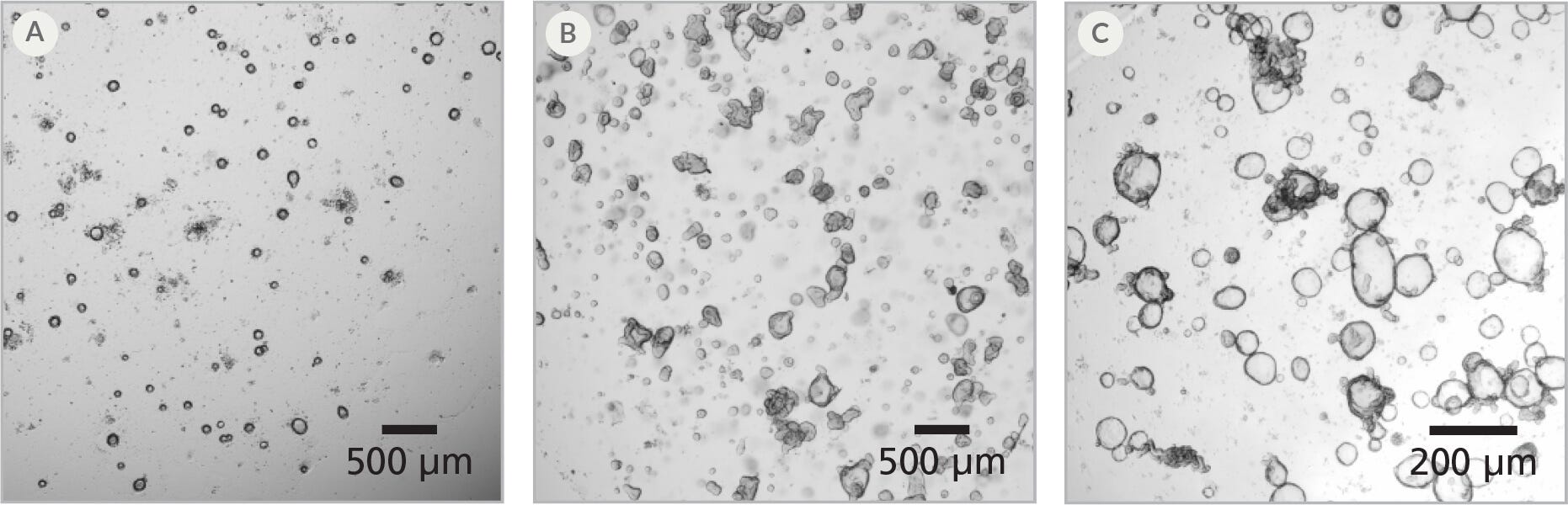
Figure 2. Intestinal Organoids Recover Quickly After Thawing
(A) One day post-thaw, organoids are small but bright and healthy-looking; (B) After 4 - 5 days, organoids have begun to form buds; (C) Healthy human intestinal organoids after 2 passages post-thaw are ready for downstream culture, differentiation, or other experiments.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration