Cell Freezing and Preservation Media
Store Your Cells and Tissues with Optimized Cryopreservation Media
Storage and cryopreservation of cells and tissues are vital parts of the workflow for biological research. Your choice of freezing medium can influence the quality of your banked cells and future experiments. Ensure high cell viability and functional stability following storage and thawing by using a reliable cryopreservation medium offered by STEMCELL Technologies.
Choose from cGMP-manufactured, protein- and/or serum-free freezing and biopreservation media to preserve your cells and tissues. For long-term storage of your cells in low temperature environments (-80°C to -196°C), opt for a ready-to-use freezing medium such as CryoStor® CS10 or mFreSR™. For short-term hypothermic preservation (2 – 8°C), you can use a preservation medium such as HypoThermosol® FRS. Explore all our cell freezing and preservation media to meet your cryopreservation workflow needs for different cell types.
Cryopreservation Basics: Protocols and Best Practices for Freezing Cells
Learn the fundamentals of cell cryopreservation and find best practices for freezing down your cells in this resource.
cGMP-Manufactured Cell Freezing and Preservation Media
Why Use cGMP-Manufactured Freezing Media?
Smooth your path through the regulatory landscape with cGMP-manufactured freezing and preservation media formulated using USP-grade ingredients. By using cGMP-manufactured freezing media, you can ensure that the products are consistently produced and controlled according to quality standards; an important consideration when you plan to move to the clinic in the future.
Defined and Serum-Free Cryopreservation Media
Why Use Serum-Free Cryopreservation Media?
Lab-made formulations of freezing medium often contain fetal bovine serum (FBS). However, FBS contains undefined components, such as growth factors and hormones, and its use in freezing media raises concerns about lot-to-lot variability and the risk of transmitting potentially infectious agents. FBS is usually not recommended for cell banking, commercial, or clinical applications, such as the production of biologicals.
Data
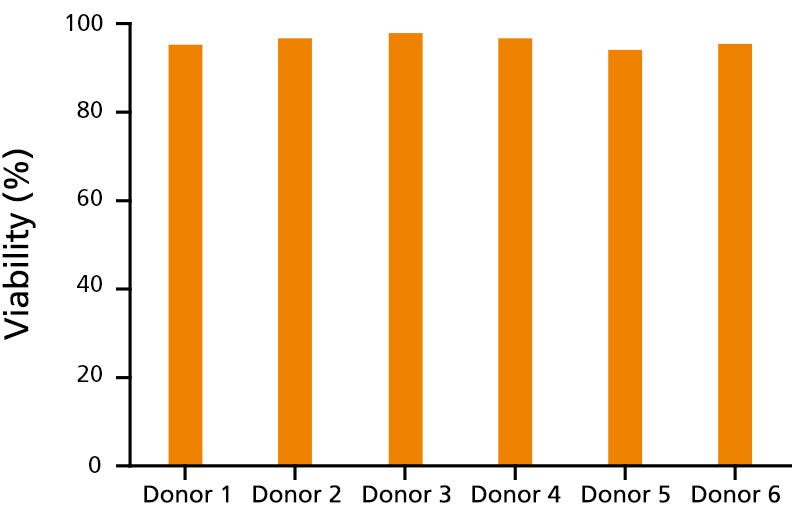
Figure 1. Immune Cells Cryopreserved in CryoStor®CS10 Show Reproducibly High Post-Thaw Cell Viability
CryoStor®CS10 effectively mitigates temperature-induced molecular cell stress responses to maximize post-thaw viability and recovery for a variety of immune cell types, including T cells (data not shown) and B cells. Here, human B cells from 6 different donors cryopreserved in CryoStor®CS10 show reproducibly high viability after thawing, as measured by Propidium Iodide staining (ranging from 94.3 - 97.9%).
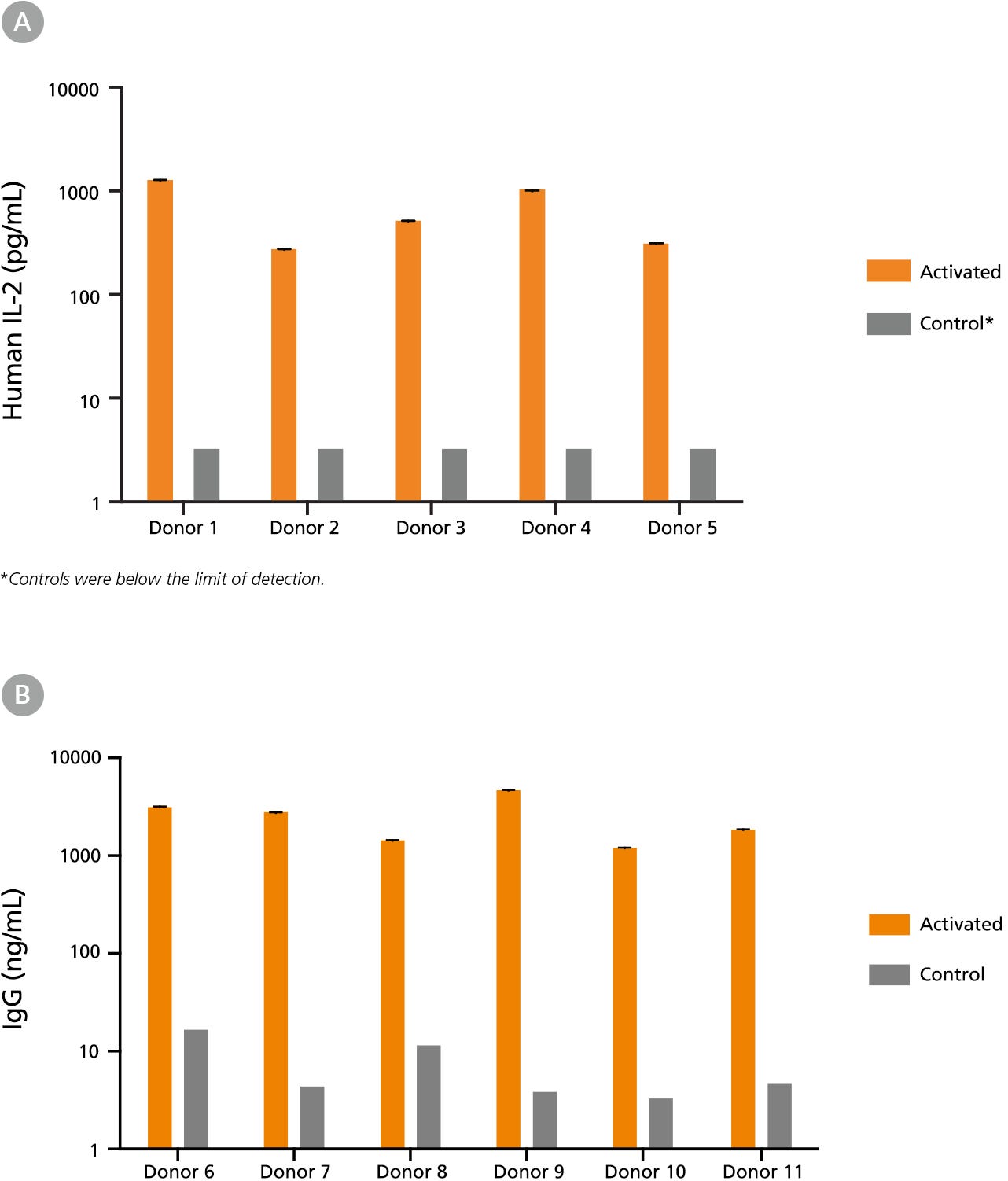
Figure 2. Immune Cells Cryopreserved in CryoStor®CS10 Retain Functionality Post-Thaw
(A) Human peripheral blood Pan-T cells cryopreserved in CryoStor®CS10 were thawed and cultured with or without the addition of T cell activating factors. Cells from Donors 1-3 were cultured in RPMI Medium supplemented with 10% FBS, with (activated) or without (control) 40 ng/mL PMA and 1 ug/mL Ionomycin for 24 hours. Cells from Donors 4-5 were cultured in ImmunoCult™-XF T Cell Expansion Medium (Catalog #10981), with (activated) or without (control) ImmunoCult™ Human CD3/CD28 T Cell Activator (Catalog #10971) for 48 hours. Supernatants were collected from the cultures, and concentrations of secreted cytokines were determined using the Human IL-2 ELISA Kit (Catalog #02006). Activation by either PMA and Ionomycin or ImmunoCult™ Human CD3/CD28 T Cell Activator led to increased secretion of IL-2 compared to unstimulated control cultures. (B) Human B cells (Donors 6 - 11) cryopreserved in CryoStor®CS10 were thawed and activated with 1 µg/mL CD40 and 100 ng/mL IL-21 for 7 days. Supernatants were collected from the cultures and immunoglobulin G (IgG) production was measured using the Human IgG ELISA Antibody Pair Kit (Catalog #01994). Compared to unstimulated control cultures, B cell activation led to increased IgG secretion.
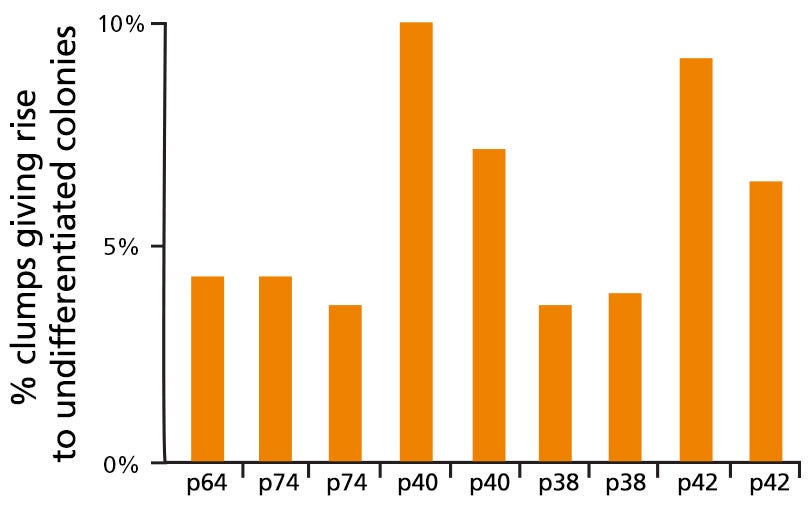
Figure 3. mFreSR™ Improves Thawing Efficiencies 5- to 10-Fold over Other Reported Methods
H9 hESCs were cryopreserved in mFreSR™ at the indicated passage number. Thawing efficiencies were analyzed by counting the number of surviving clumps after thawing.
Related Products to Complete Your Freezing & Thawing Workflow
Complete your cryopreservation workflow by preserving your cells and tissues in sterile, self-standing cryogenic vials of different sizes and cap colors. Or obtain consistent freezing profiles using an alcohol-free container ideal for the cryopreservation of most cells and cell lines. Explore products for sterile cryostorage and freezing below:
For downstream processing, researchers can thaw their cryopreserved cells using the ThawSTAR® CFT2 Automated Thawing System—a water-free instrument that delivers cell thawing profiles similar to those of a water bath. Conveniently thaw your cells in the biosafety cabinet, in ~2.5 minutes, while you prepare for the next step in your experiment.
Standardize Your Cell Thawing Process
Increase confidence in your cell thawing workflow by ensuring sample sterility and consistent thawing performance with ThawSTAR® CFT2—a sensor-based automated thawing system.
Step-by-Step Cell Thawing Demonstration
Follow this step-by-step protocol video to see how ThawSTAR® CFT2 could fit in your laboratory’s cell thawing workflow.
Protocols and Technical Tips for Freezing and Thawing Cells
Cryopreservation and Thawing of ES/iPS Cells
Cryopreservation and thawing of human ES or iPS cells using either mFreSR™, CryoStor® CS10, or FreSR™-S.
How to Thaw Frozen Primary Cells
Step-by-step protocol for thawing frozen primary cells.
References
- Fujioka T, et al. (2004) A simple and efficient cryopreservation method for primate embryonic stem cells. Int J Dev Biol 48(10): 1149-54
- Ha SY, et al. (2005) Cryopreservation of human embryonic stem cells without the use of a programmable freezer. Hum Reprod 20(7): 1779-85
- Ji L, et al. (2004) Cryopreservation of adherent human embryonic stem cells. Biotechnol Bioeng 88(3): 299-312
- Ware CB, et al. (2005) Controlled-rate freezing of human ES cells. Biotechniques 38(6): 879-80, 882-3