How to Cryopreserve Intestinal Organoids
This protocol describes how to freeze mouse or human intestinal organoids cultured in IntestiCult™ Organoid Growth Medium (Mouse), IntestiCult™ Organoid Growth Medium (Human), or IntestiCult-SF™ Organoid Growth Medium (Human) in CryoStor® CS10 cryopreservation medium. For optimal results, cryopreservation is best performed midway through or late in the passage, when organoids are near or at their maximal size.
Materials
- CryoStor® CS10 (Catalog #07930)
- Gentle Cell Dissociation Reagent (Catalog #100-0485)
- PBS without magnesium or calcium (e.g. D-PBS Without Ca++ and Mg++, Catalog #37350)
- DMEM/F-12 with 15 mM HEPES (e.g. Catalog #36254)
- 15 mL conical tubes (e.g. Falcon® Conical Tubes, Catalog # 38009)
- Cryovial (e.g. Cryogenic Vials with Green Caps, Catalog #38053)
Preparation
- Place DMEM/F-12 with 15 mM HEPES, PBS without magnesium or calcium, Gentle Cell Dissociation Reagent (GCDR), and CryoStor® CS10 to cool on ice.
- Retrieve the plate(s) containing the organoids that are being cryopreserved.
Note: Intestinal organoids are suitable for cryopreservation after two passages from primary culture, or from cryopreserved organoids that have been thawed and cultured. Large cystic or budded organoids result in a higher yield of viable fragments than small, dark, or collapsed organoids. Human intestinal organoids and mouse colonic organoids can be cryopreserved from day 5 post-passage onwards (Figure 1), while mouse small intestinal organoids can be cryopreserved day 3 post-passage onwards (Figure 2).
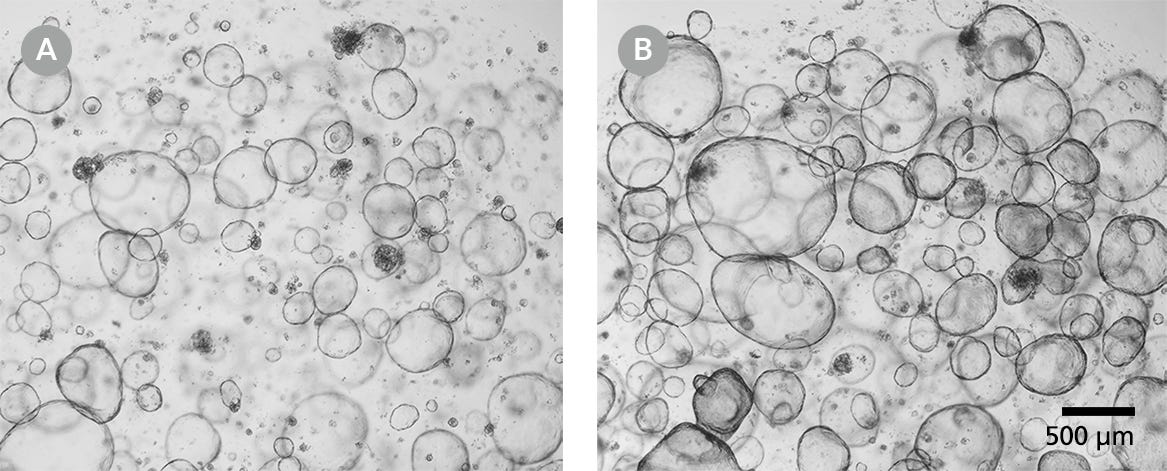
Figure 1. Human Intestinal Organoids for Cryopreservation
Human intestinal organoids should be cryopreserved from day 5 post-passage onwards. (A) Human colonic organoids after 5 days of culture and (B) after 7 days of culture. Light microscope visualization (4X) of intestinal organoids cultured in domes of 1:1 Matrigel® Matrix and IntestiCult™ Organoid Growth Medium and incubated at 37°C and 5% CO2.
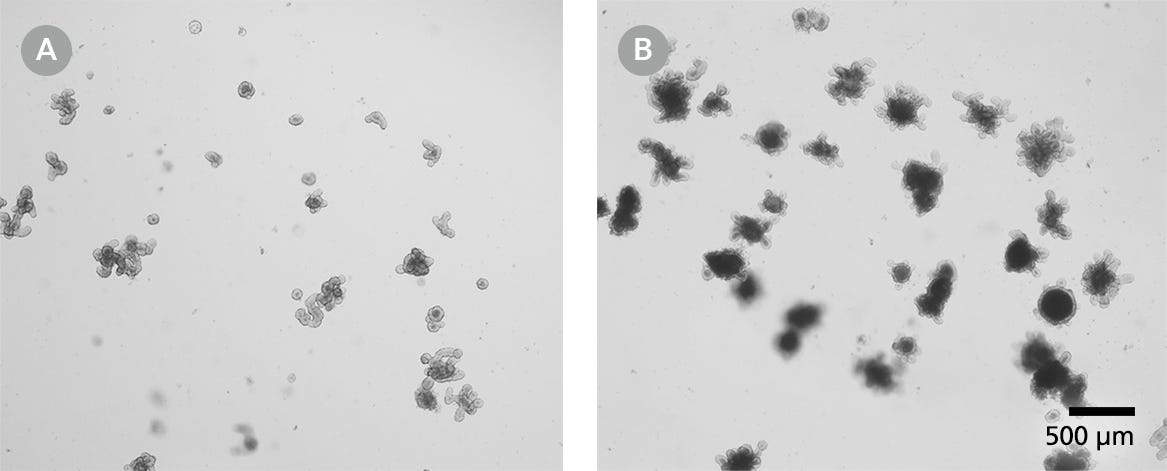
Figure 2. Mouse Intestinal Organoids for Cryopreservation
Mouse intestinal organoids should be cryopreserved from day 3 post-passage onwards. (A) Small intestinal organoids after 3 days of culture and (B) after 10 days of culture. Light microscope visualization (4X) of intestinal organoids cultured in domes of 1:1 Matrigel® Matrix and IntestiCult™ Organoid Growth Medium and incubated at 37°C and 5% CO2.
Protocol
- Using an inverted microscope, count the number of organoids found in each well. Combine the contents of multiple wells as needed to achieve 200 organoids per cryovial to be filled.
- Remove IntestiCult™ Organoid Growth Medium from each well containing organoids. Replace it with 1 mL of cold (2 - 8°C) GCDR or PBS.
Note: We recommend using GCDR to assist with dissociation, but PBS may be used instead if desired.
- Break up the Corning® Matrigel® Matrix by pipetting up and down 2-3 times with a pre-wetted 1000 μL pipette tip. Transfer suspensions containing 200 organoids, combining wells if necessary, to a single 15 mL conical tube.
- Wash each well with 1 mL of cold (2 - 8°C) GCDR or PBS by pipetting up and down 2-3 times with a pre-wetted 1000 μL pipette tip and transfer to the 15 mL conical tube.
- Pellet the organoids by centrifuging at 290 x g for five minutes at 2 - 8°C. Carefully remove and discard the supernatant. To avoid aspirating the organoid pellet, remove the last 1ml of medium by using a pre-wetted P1000 pipette tip followed by a pre-wetted P200 pipette tip.
- Resuspend organoid pellet in 1mL of cold DMEM/F-12. Pipette up and down approximately 15 times for small intestinal organoids, or approximately 25 times for colonic organoids, in order to fragment them prior to cryopreservation. The size of resulting fragments can be monitored if desired, by transferring 10 µL cell suspension to a haemocytometer or empty culture dish, and examining under the microscope. Organoids should be fragmented. If too little pipetting was performed, intact organoids may still be present, but if too much pipetting was performed, an excess of single cells and debris may be observed. Please note that cystic organoids fragment more quickly than budded organoids.
Note: The size of resulting fragments can be monitored if desired, by transferring 10 µL of cell suspension to a haemocytometer or empty culture dish, and examining under a microscope. Organoids should appear fragmented. Intact organoids may still be present if too little pipetting was performed. If too much pipetting was performed, an excess of single cells and debris may be observed. Cystic organoids fragment more rapidly than budded organoids.
- Add an additional 9 mL of cold DMEM/F-12 with 15 mM HEPES to wash the organoid fragments. Centrifuge the suspension at 200 x g for five minutes at 2 - 8°C then carefully remove and discard the supernatant. To avoid aspirating the organoid pellet, remove the last 1ml medium by using a pre-wetted P1000 pipette tip followed by a pre-wetted P200 pipette tip.
- Gently resuspend the organoid pellet in cold (2 - 8°C) CryoStor® CS10 freezing medium using 1 mL of freezing medium per cryovial of 200 fragmented organoids.
- Using the same pipette tip, transfer the fragmented organoids suspended in CryoStor® CS10 to a labeled cryovial. Place the cryovial in a freezing container with 500 mL of isopropyl alcohol, or in an IPA-free freezing container.
- Transfer the freezing container to a -80°C freezer for 24 hours then transfer the cryovial to liquid nitrogen (-135°C) for long-term storage. Long-term storage at -80°C is not recommended.
Note: Work quickly to avoid prolonged exposure of non-frozen organoids to CryoStor® CS10.
Tips for Cryopreservation of Organoids in CryoStor® CS10
- Prior to cryopreservation, ensure that the organoids are not overgrown and that the lumen is not too dark. The cellular debris shed by the lumen will decrease the viability of the cultures post-thaw. While blebbing structures (Figure 3B) are acceptable for organoid passaging, they are not ideal for cryopreservation. Blebbing indicates the deterioration of an organoid, where the epithelium falls apart into single cells and appears as ruffled membranes with areas of single cells.
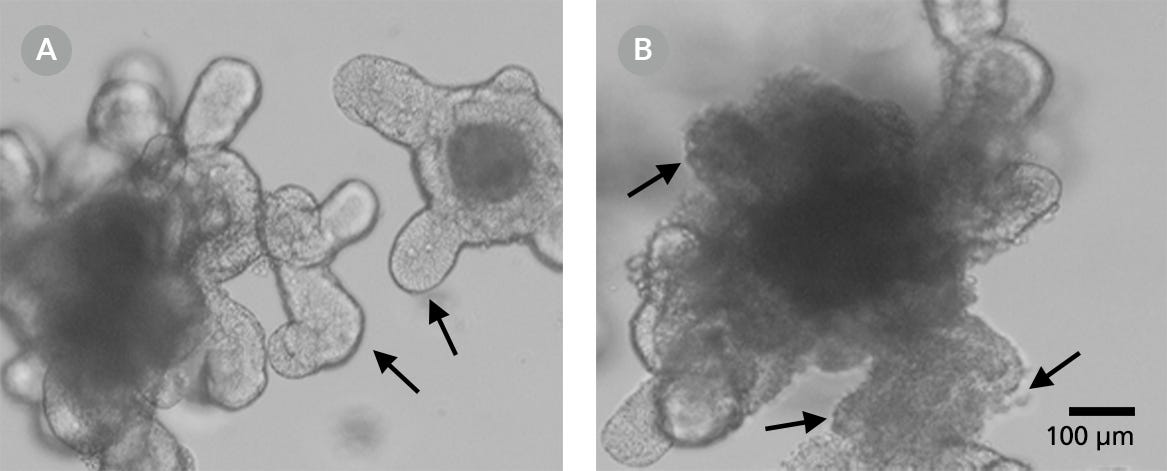
Figure 3. A Smooth Surface is Indicative of a Healthy Organoid, whereas Blebbing Indicates Deterioration in Organoid Quality
Representative images of smooth epithelium (A) and blebbing (B). Green arrows = smooth epithelium on buds. Red arrows = deteriorating epithelium with visible single cells (red arrows).
- 200 organoids per cryovial is the optimal density for cryopreservation and post-thaw viability. Fewer organoids (80-100 organoids) may be used, but 200 is sufficient to allow for some organoid loss during processing.
- To re-freeze organoids thawed organoids, passage at least twice before freezing. When organoids are subjected to rough environmental conditions (lack of medium changes, dimethyl sulfoxde exposure) their growth will slow. Organoids need to recover prior to expansion for subsequent cryopreservation.
- The stability of organoids cropreserved in CryoStor® CS10 is 6 months at -135°C. Long-term storage at -80°C is not recommended.
- It is possible to freeze down organoids on days 2-3 if they have healthy morphology. However, they may expand with less efficiency. Day 5-6 is optimal for all human intestinal organoids and for mouse colonic organoids, whereas day 3 is optimal for mouse small intestinal organoids. This is because there are sufficient stem cells present to develop into new organoids).
- It is not recommended to cryopreserve whole organoids as intact structures. Organoids will break down and fragments will die off during the thawing process, so you will lose stem cell components.
- Please note that we have not investigated the viability between organoids frozen at mid- or late-passage. Our suggestion is for customers to evaluate both options, and compare viability, if feasible or if they are concerned.
Frequently Asked Questions
Can I use cryopreservation medium other than CryoStor® CS10?
We have only tested CryoStor® CS10 in-house. Other cryopreservation media may be suitable, but would need to be tested.
How long after derivation should I biobank intestinal organoids?
After derivation, it is good practice to expand organoid lines for two passages so that they can reach their maximal expansion rate, and then to biobank them. Cryopreserving at this early stage guards against losing a line to contamination, or late-passage phenotypic changes.
For how long can intestinal organoids be kept frozen?
We don't have a time limit for long-term storage, however, organoids should be stable for at least 6 months. Long-term storage at -80° C is not recommended.
How many times should I passage thawed organoids before freezing again?
Organoids should be expanded for two passages in order to allow them to recover from the freeze-thaw process. Upon the first passage post-thaw, cultures should be passaged using a 1:1 ratio, and upon the second, they can be passaged using a 1:4 - 1:6 ratio. They can then be refrozen, or used for downstream experiments.
Will organoids recover if they have been degraded before or after cryopreservation?
We have been able to show that as long as there are healthy, single, intestinal stem cells in the culture, previously degraded organoids recover after one passage (Figure 4). These organoids generally take an additional three to five days to establish and will likely require multiple passages to generate an adequate population of organoids. Addition of 10 µM Y-27632 can be beneficial in establishing organoids grown from single cells or very small clumps. It only needs to be added during seeding and can be removed at the first media change.
Troubleshooting
Few organoids grow after thaw.
This can be due to organoid loss during handling. In order to guard against this: 1) Always use pipette tips that have been pre-wetted with PBS, GCDR or DMEM + 0.1% BSA when handling organoids, and 2) Take care not to aspirate pelleted organoids, by removing the last 1ml medium by using a P1000 pipette tip and then a P200 pipette tip.A low number of organoids can also be due to poor viability (see below).
There is poor viability after thaw.
Ensure that organoids are at least 5 days post-passage upon freezing, that they are fragmented adequately, that they are frozen at the recommended density, and that a validated freezing medium is used. Ensure that organoids are thawed according to our protocol, in particular ensuring that the cryovial is warmed in a 37° water bath for just enough time to melt the frozen ice and no longer, and that 1mL of DMEM/F-12 + 1% BSA is added directly to the cryovial and gently mixed with before transferring the cells to a larger tube and topping up the medium. In order to assist cultures in recovering from thaw, passage them at a 1:1 ratio on the first passage, and a 1:4 - 1:6 ratio on the second passage.
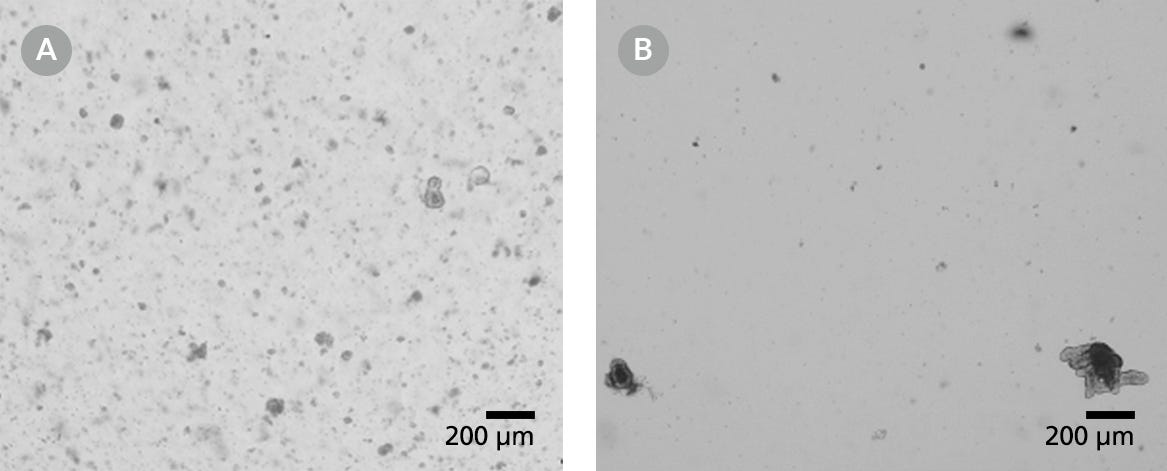
Figure 4. Light Microscope Visualizations of Intestinal Organoid Cultures from Frozen Organoids That Had Been Degraded Either Before or After Cryopreservation
Organoids cultured in domes of 1:1 Matrigel® Matrix and IntestiCult™ Organoid Growth Medium are shown following incubation at 37°C and 5% CO2 at (A) Passage 0, Day 5 and (B) Passage 1, Day 6.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration