How to Differentiate hiPSCs into Colonic Organoids Using STEMdiff™
Colorectal cancer is the leading cause of cancer death in men aged under 50 and the second-leading cause in women under 50.1 Scientists currently rely on immortalized cell lines and animal models to translate knowledge from basic research into clinical therapeutics; however, neither of these models accurately represent the complexity of tumor biology. Colon (or large intestinal) organoids provide researchers with a physiologically relevant cell model and constitute a valuable experimental tool for studying large intestine cell biology, modeling diseases, such as colorectal cancer, or cytotoxicity screening of novel therapeutics in drug discovery.2,3
In this protocol, colon organoids are generated by differentiating human induced pluripotent stem cells (hiPSCs) through a multi-step process from definitive endoderm to posterior hind-gut endoderm and eventually into colon organoids through BMP signaling.4 The resulting human colonic organoids (HCOs) express colon-specific markers, including colonic transcription factor SATB2, a-carbonic anhydrase II (CA-II), a-carbonic anhydrase IV (CA-IV), goblet cell markers Mucin-2 (MUC2) and Mucin-5B (MUC5B), as well as upregulation of posterior HOX genes (HOXA10, HOXA13, and HOXD13).
Below, we describe a step-by-step guide for preparing a HCO growth medium and for generating hiPSC-derived colonic organoids using the HCO growth medium in combination with STEMdiff™ Intestinal Organoid Kit. These colonic organoids are distinct from small intestinal organoids, which can also be generated using the STEMdiff™ Intestinal Organoid Kit, as demonstrated in Figures 2 - 4 below.

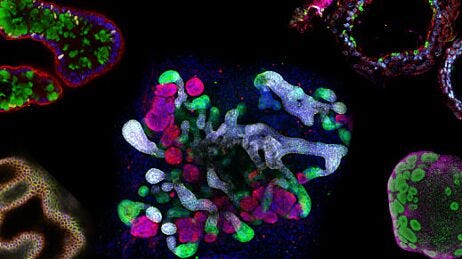
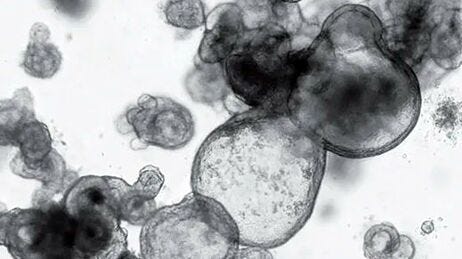
Figure 1. Generation of Human Colonic Organoid Cultures Using STEMdiff™ Intestinal Organoid Kit
Human PSC cultures progress through a four-stage differentiation process to generate human colonic organoids. (B-D) Matured human intestinal organoids and (E-F) human colonic organoids (days in parentheses indicate days post-embedding in a given passage). After multiple passages, the organoids generally exhibit less sinking within the matrix dome and a lower proportion of mesenchymal cells.
Materials
- STEMdiff™ Intestinal Organoid Kit (Catalog #05140)
- DMEM/F-12 + 15 mM HEPES (Catalog #36254)
- L-Glutamine (Catalog #07100)
- Human Recombinant EGF (rh-EGF) (Catalog #78006)
- Human Recombinant BMP2 (rh-BMP2) (Catalog #78004)
- CHIR99021 (Catalog #72052)
- NeuroCult™ SM1 Neuronal Supplement (Catalog #05711)
- Corning® Matrigel® Growth Factor Reduced (GFR), Phenol Red-Free (Corning Catalog #356231)
Preparation
Prepare human colonic organoid (HCO) growth media as shown in Table 1 using sterile technique.
Table 1. Concentrations of Components Required to Prepare HCO Growth Medium
Protocol
- Generate mid/hindgut spheroids following the STEMdiff™ Intestinal Organoid Kit Technical Manual (harvested on Day 7/8).
- Pool the spheroid suspension and embed the spheroids into approximately 100% Matrigel® domes at a density of 70 - 100 free-floating spheroids per dome. Allow the Matrigel® to solidify at 37°C for approximately 10 minutes.
- Add 0.5 mL of complete HCO growth medium with spiked-in BMP2 (100 ng/mL) carefully to the side of each well without disturbing the dome. Incubate at 37°C with 5% CO2 and 95% humidity.
- After 3 days, perform a complete media change and add 0.5 mL of complete HCO growth medium (without BMP2) to each well. Incubate at 37°C with 5% CO2 and 95% humidity.
- Perform a media change 3 times per week and passage the organoids into fresh Matrigel® every 10 - 14 days according to the proper split ratio (approximately 80 - 100 organoids per dome).
Note: For more information on passaging organoids, refer to section 7.2 (“Passaging Human Intestinal Organoids”) from the STEMdiff™ Intestinal Organoid Kit Technical Manual.Note: Antibiotics (e.g. 50 µg/mL gentamicin) can be added into the growth media after the 1st passage.
- Colorectal organoids should be fully established by the end of the 3rd passage and are ready for characterization or further downstream applications.
Note: A decline in culture growth rate may occur following multiple passages, this may be due to reduced mesenchymal and stem cell populations.

Figure 2. Morphology of hiPSC-Derived Small and Large Intestinal Organoids from Various Cell Lines
STEMdiff™ Intestinal Organoid Kit facilitates directed differentiation of PSCs to form human (A-D) small intestinal organoids and (B-H) large intestinal organoids. The organoids exhibit a polarized epithelial monolayer surrounding a hollow lumen and are associated with a mesenchymal cell population. Pictured are passage 2 human small and large intestinal organoids generated using this kit. Scale bar = 0.2 mm.

Figure 3. Human iPSC-Derived Colonic and Small Intestinal Organoids Express Key Genetic Markers of Respective Tissues
Differentiated hiPSC-derived colonic organoids (HCOs) exhibited expression of key intestinal markers such as CDX2 and LGR5, in addition to an upregulation in key genetic markers of the large intestine and the associated mesenchyme with increased goblet cell markers (MUC2 and MUC5B), colon transcription factor SATB2, α-carbonic anhydrases CA2 and CA4, and the posterior homeobox (HOX) markers found in the hindgut. Human intestinal organoids (HIOs) differentiated from the same cell lines (H9, 3A, H1, and 1C) demonstrated downregulation for the colonic markers and upregulation for the anterior HOX markers of the fore/midgut. Total healthy adult colon and small intestine were assessed as positive controls. Marker levels were assessed by RT-qPCR and are normalized expression levels relative to the housekeeping (HK) genes Ubiquitin C and TATA-box binding protein.

Figure 4. hiPSC-Derived Colonic Organoids Exhibit Distinct Features of the Colon Epithelium
hiPSC-derived (A-C) colonic organoids demonstrate increased markers for the hindgut such as MUC2 (a pan-goblet cell marker), MUC5B (a colon-specific goblet cell marker), and SATB2 (a colorectal differentiation marker) when compared to hiPSC-derived (D-F) intestinal organoids.
References
- Siegel RL et al. (2024) Cancer statistics. CA Cancer J Clin 74(1): 12–49.
- Crespo M et al. (2017) Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med 23(7), 878–884.
- Yoshida S et al. (2020) Generation of intestinal organoids derived from human pluripotent stem cells for drug testing. Sci Rep 10(1), 5989.
- Múnera JO et al. (2017) Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell 21(1), 51–64.e6.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration