Frequently Asked Questions on STEMdiff™ Intestinal Organoid Kit
Find answers to frequently asked questions (FAQs) about culturing intestinal organoids using the STEMdiff™ Intestinal Organoid Kit.
General Protocols and Passaging
Can antibiotics be added to STEMdiff™ Intestinal Organoid Growth Medium (OGM) media for organoid maintenance?
For the STEMdiff™ Intestinal Organoid Kit, we do not recommend the use of antibiotics in the early stages of culture (Stages 1 - 2). While not advisable, antibiotics could be added to spheroids if desired after the first passage post-embedding. Once organoids are fully formed (> P2), it should be safe to add any standard antibiotic to the medium (e.g. gentamicin at a final concentration of 50 µg/ml).
What is the benefit of clump versus single-cell passaging at the start of the differentiation protocol?
Clump passaging results in the formation of larger mid-/hindgut spheroids, which ultimately lead to a higher differentiation efficiency, and is more robust. It is recommended that customers optimize their seeding densities to achieve optimal differentiation results if starting with single cells.
When should spheroids be removed and embedded between Days 5 - 9 of the Stage 2 protocol?
Mid-/hindgut spheroids released between Day 5 - 9 are capable of giving rise to human small intestinal organoids. Note that the duration that the cells are exposed to mid-/hindgut medium will steer the regional identity of the developing small intestinal organoids to an identity similar to duodenum (shorter exposure) or ileum (longer exposure). For reproducible results, it is recommended to consistently harvest and initiate human intestinal organoid cultures using mid-/hindgut spheroids generated on the same day of differentiation. Day 8 is often when the most budding is observed, but this depends on the cell line.
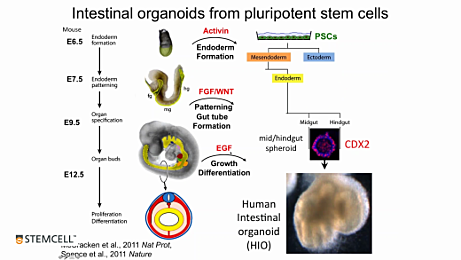
What is the difference between a spheroid and an organoid?
Spheroids are budded from the monolayer during Stage 2 and embedded into Matrigel® at P0. After 7 - 10 days of culture, spheroids differentiate and are thereby referred to as human intestinal organoids (HIOs). Mid-/hindgut spheroids are composed of polarized epithelium expressing CDX2, E-Cadherin, and EpCam. In addition, they contain mesoderm-derived cell types that express vimentin and CDX2, but not EpCam. The mesenchyme can be seen through the microscope and often envelops the spheroids (Figure 4 on the STEMdiff™ Intestinal Organoid Kit Product Landing Page). HIOs express intestinal, goblet, and enteroendocrine cell-specific markers, such as CDX2, MUC2, EpCam, CHGA, SOX9, and KRT20. They also express mesenchymal markers desmin and vimentin. The presence of Ki67 confirms cell proliferation and the presence of putative intestinal stem cells, which enable long-term organoid culture (Figure 5 on the STEMdiff™ Intestinal Organoid Kit Product Landing Page).
Can Stage 1 be extended by one day if CXCR4/SOX17 or FOXA2/SOX17 expression is low?
No, we advise against this. We tested longer and shorter durations of Stage 1, which both resulted in lower efficiencies of spheroid formation during Stage 2.
Can you extend Stage 2 for more than 9 days to obtain more spheroids?
Yes, as long as the cell monolayer is still giving rise to spheroids. However, it has been observed with 5 different PSC cell lines that after 9 days in differentiation, the Stage 2 monolayer no longer produces spheroids.
Is there control over the size of spheroids between Days 5 - 9?
No, but earlier days generally produce smaller spheroids.
Is it possible to embed fewer spheroids into a smaller dome?
It may be possible but we haven’t tested it. It may be more challenging to initiate organoid cultures from smaller spheroids.
If spheroids are held loosely by the monolayer and have not fully detached, can they be forced off by washing?
This is not a good idea as you may carry over unwanted cells from the monolayer.
Can I increase the initial seeding density between different cell lines?
Yes, the Technical Manual (Generation of Human Intestinal Organoids Using STEMdiff™ Intestinal Organoid Kit) suggests seeding a range of clump densities (e.g. 4000, 5000, and 6000 clumps per well in a 24-well plate). Optimizing the initial seeding density is critical for reproducible and robust spheroid formation at Stage 2 of differentiation. Customers can try greater than 6000 clumps per well if needed.
What kind of variability can be expected between cell lines?
There is intrinsic variability between cell lines in the efficiency of differentiation into mid-/hindgut progenitors. We have tested multiple embryonic stem cell (ESC) and induced pluripotent stem cell (iPSC) lines and observed similar effects (Figure 3 in STEMdiff™ Intestinal Organoid Kit Brochure).
Can a 1:1 Matrigel® dilution be used? Would the epithelium be thicker in pure Matrigel®?
Both dome formation and morphology are better preserved in a pure Matrigel® dome (100% Matrigel®). We don't recommend a 1:1 dilution for hPSC-derived organoids, especially at early passages, due to the presence of outgrowing mesenchyme, which digests and re-models its environment. Diluted Matrigel® accelerates this remodeling process, resulting in the intestinal organoids sinking to the bottom of the well and attaching to the plastic, which is not desirable.
Culture Formats
What is the best way to scale down cultures into a 96-well plate?
It is better to establish the organoids first in a 24-well plate and then scale down the format. Customers can use the 24-well seeding densities as a starting point and scale appropriately, however, we haven't done any of this optimization in-house.
For the generation of mid-/hindgut spheroid cultures, they can be scaled down into 96-well plates. However, for hPSC-derived organoids, we would advise starting with 24-well plates to generate enough mid-/hindgut spheroids from multiple samples/donors, which will allow them to embed into multiple replicate Matrigel® domes in a smaller format, e.g. 96-well.
Can this culture be carried out in larger volumes, such as T-75 or T-175 flasks?
We have not tested flasks such as T-75 or T-175. Further optimization of the cell seeding density for the scale-up would be required. The recommended seeding density for 24-well plates is between 4000 - 6000 clumps per well (= 1.9 cm2), which corresponds to a T-75 flask of a range between approximately 160,000 - 240,000 clumps per flask. Customers should initiate differentiation (Stage 1 medium) when the density of hPSCs reaches 85 - 90%.
Have you tested suspension cultures instead of dome cultures?
We have not optimized the suspension culture format. Matrigel® domes are the standard culture format in the field, and we expect to see the best morphologies of intestinal organoids in domes rather than suspension.
Performance and Characterization
How many spheroids do you typically get from one well at Stage 2 during Days 4 - 9?
At earlier time points (Days 4 - 5), fewer spheroids are shed. Later on, it is common to get at least 50 spheroids per well, with sometimes as many as 300 - 500 per well toward Day 8.
What is the difference between the organoids generated using the STEMdiff™ Intestinal Organoid Kit, and those from primary biopsies cultured using IntestiCult™ Organoid Growth Medium (Human)?
Organoids derived from primary human biopsies using IntestiCult™ Organoid Growth Medium (Human) are less differentiated, as demonstrated by the positive staining of Ki67 (proliferation marker), EpCAM (epithelial marker), and Vilin (luminal marker) (Figure 2 on the IntestiCult™ Organoid Growth Medium (Human) Product Landing Page). Organoids derived using STEMdiff™ Intestinal Organoid Kit contain differentiated cell types, as well as stem and progenitor types (Figure 5 in the STEMdiff™ Intestinal Organoid Kit Brochure, Figure 7 in the Scientific Poster). These organoids also contain mesenchyme which contributes to the niche factors.
Does the apical surface, which faces inwards to the organoid, secrete mucus?
Yes, because the organoids contain goblet cells, as evidenced by the fact that we can detect mucin 2 (MUC2) protein within the cytoplasm and at the apical surface of goblet cells (Figure 5B in the STEMdiff™ Intestinal Organoid Kit Brochure).
Why are smaller spheroids less desirable than larger spheroids?
It is more difficult to establish organoids from smaller spheres (under 75 μm); larger spheroids have a higher efficiency in forming organoids.
Do PSC-derived organoids display a specific organoid phenotype?
Different cell lines display different organoid morphologies (mixed morphologies of cystic and budding organoids, or either cystic or budding). Over time and multiple passages in STEMdiff™ Intestinal OGM, the cystic organoids may become more prevalent than budding organoids.
Have any comparative studies been carried out looking at the differences in expression of mesenchymal markers between the definitive endoderm and differentiated intestinal organoid stages? Have any other mesenchymal markers been observed, other than vimentin and CDX2?
Many more mesenchymal cells can be observed at earlier passages (Figure 2B in the STEMdiff™ Intestinal Organoid Kit Brochure). The characterization of brachyury, vimentin, ACTA2, and desmin markers in the DE stage versus other stages (organoids) was presented in STEMdiff™ Intestinal Organoid Kit Brochure (Figures 4A and 5A).
Has the mesenchyme proportion been tested at every passage?
There is a decrease of mesenchyme due to fragmentation of organoids at each passage. After 40+ passages, some mesenchyme is still present.
What is the highest number of passages tested in-house?
The highest number of in-house passages tested is 40 passages with one freeze/thaw cycle. We have successfully maintained hPSC-derived HIOs for more than one year in culture (n = 3) by passaging them every 7 - 10 days.
Downstream Applications
What is the best way to scale up organoid cultures for high-throughput screening (HTS) applications?
To scale up organoid cultures, it is advised to generate multiple Matrigel® domes per well (e.g. in a 6-well plate), as well as to increase the number of organoids per Matrigel® dome (up to 100 organoids per dome). In this scenario, we recommend performing medium changes three times a week, instead of only twice a week.
Can organoids generated using this kit be further differentiated into mature organoids?
Organoids generated using STEMdiff™ Intestinal Organoid Kit are considered to be fetal in nature and not as matured as in fully developed primary intestinal tissue. However, these organoids can be further differentiated to express markers of differentiated intestinal cells, such as goblet cells and enteroendocrine cells, as an end-point assay. Organoids must first be passaged at least 2 - 3 times or cultured for at least 30 days during Stage 3. Cultures are then maintained for up to 2 weeks with regular media changes using the STEMdiff™ Intestinal Organoid Growth Medium to allow further differentiation of organoids. Disruption will reactivate the stem cells; by leaving the cultures without passaging, we can allow the organoids to further differentiate. We do not have a recommendation for differentiation media.
Cryopreservation
Is it possible to cryopreserve PSC-derived intestinal organoids?
Human PSC-derived intestinal organoids can be frozen as early as passage 3 once they have fully established and display mature, budding, or cystic morphologies. Please refer to Section 8.1.1 of the Technical Manual (Generation of Human Intestinal Organoids Using STEMdiff™ Intestinal Organoid Kit) for a detailed protocol.
Are there any suggestions regarding the preservation of spheroids for later use? Have you tested any short-term or long-term cryopreservation solutions specifically for spheroids?
Although it has not been extensively tested or validated, we expect that the mid-/hindgut spheroids can be cryopreserved. One reason this may not succeed could be due to a low number of spheroids, which is usually the case with the daily harvest of spheroids. Instead of daily spheroid harvests, our recommendation is to increase the number of spheroids on one single day by harvesting released spheroids and subsequently adding them back to the monolayer cultures with the fresh medium change. This way, you can cryopreserve the spheroids on a single day, rather than cryopreserving just a few spheroids daily and running into the challenges associated with low spheroid quantities in the early days.
Related Resources
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration