Mobilized Human Peripheral Blood Mononuclear Cells, Frozen
Primary human cells, frozen
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
Overview
Donors are mobilized with:
• G-CSF: a maximum of 10 µg/kg/day of granulocyte colony-stimulating factor for 3 - 5 days prior to collection (Catalog #70049)
• Plerixafor: a maximum of 0.24 mg/kg plerixafor for 1 day prior to collection (Catalog #70074)
• G-CSF and Plerixafor: a maximum of 10 µg/kg/day of G-CSF for 3 - 5 days prior to collection and a maximum of 0.24 mg/kg of plerixafor for 1 day prior to collection (Catalog #70072)
Isolated from leukapheresis samples using density gradient separation or red blood cell lysis and cryopreserved in animal component-free CryoStor®CS10 medium (Catalog #07930), cells are obtained using Institutional Review Board (IRB)-approved consent forms and protocols. Additional documentation and high-resolution HLA typing (Class I and Class II alleles and CMV status) are available upon request. Acid-citrate-dextrose solution A (ACDA) is added as an anticoagulant. Donor specifications (e.g. BMI category, smoking status, ethnicity, etc.) can be requested in the comment box above, after selecting from the product options. Donors are screened for HIV-1, HIV-2, hepatitis B, and hepatitis C.
Certain products are only available in select territories. Please contact your local sales representative or Product & Scientific Support at techsupport@stemcell.com for further information.
Browse our Frequently Asked Questions (FAQs) on Primary Cells.
Data Figures
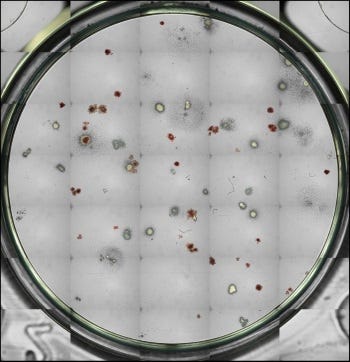
Figure 1. Cryopreserved G-CSF Mobilized Human Peripheral Blood Mononuclear Cells Generate Hematopoietic Colonies in CFU Assays
Cryopreserved G-CSF Mobilized Human Peripheral Blood Mononuclear Cells (Catalog #70049) were thawed, plated at a concentration of 1 x 10^4 viable cells/dish and cultured in MethoCult™ Optimum Medium (Catalog #04034) for 14 days. Shown are colonies produced by hematopoietic progenitor cells within the sample, imaged with STEMvision™.
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Resources and Publications
Educational Materials (13)
Related Products
-
 ThawSTAR® CFT2 Automated Thawing System
ThawSTAR® CFT2 Automated Thawing SystemAutomated cell thawing system for consistent thawing performance
-
 Human Bone Marrow Mononuclear Cells, Frozen
Human Bone Marrow Mononuclear Cells, FrozenPrimary human cells, frozen
-
 Human Cord Blood Mononuclear Cells, Frozen
Human Cord Blood Mononuclear Cells, FrozenPrimary human cells, frozen
-
 Human Peripheral Blood Mononuclear Cells, Fro...
Human Peripheral Blood Mononuclear Cells, Fro...Primary human cells, frozen
-
 Mobilized Human Peripheral Blood CD34+ Cells,...
Mobilized Human Peripheral Blood CD34+ Cells,...Primary human cells, frozen
Item added to your cart

Mobilized Human Peripheral Blood Mononuclear Cells, Frozen
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL QUALITY INFORMATION, REFER TO WWW.STEMCELL.COM/COMPLIANCE.














