ImmunoCult™-XF
cGMP, serum-free, and xeno-free medium for the expansion of human T cells
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
Overview
ImmunoCult™-XF can be used individually or in combination with ImmunoCult™ Human CD3/CD28 T Cell Activator or ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator for a gentle, bead-free activation of T cells. For more information on the expansion of T cells with ImmunoCult™, please explore the Product Information Sheet.
Data Figures
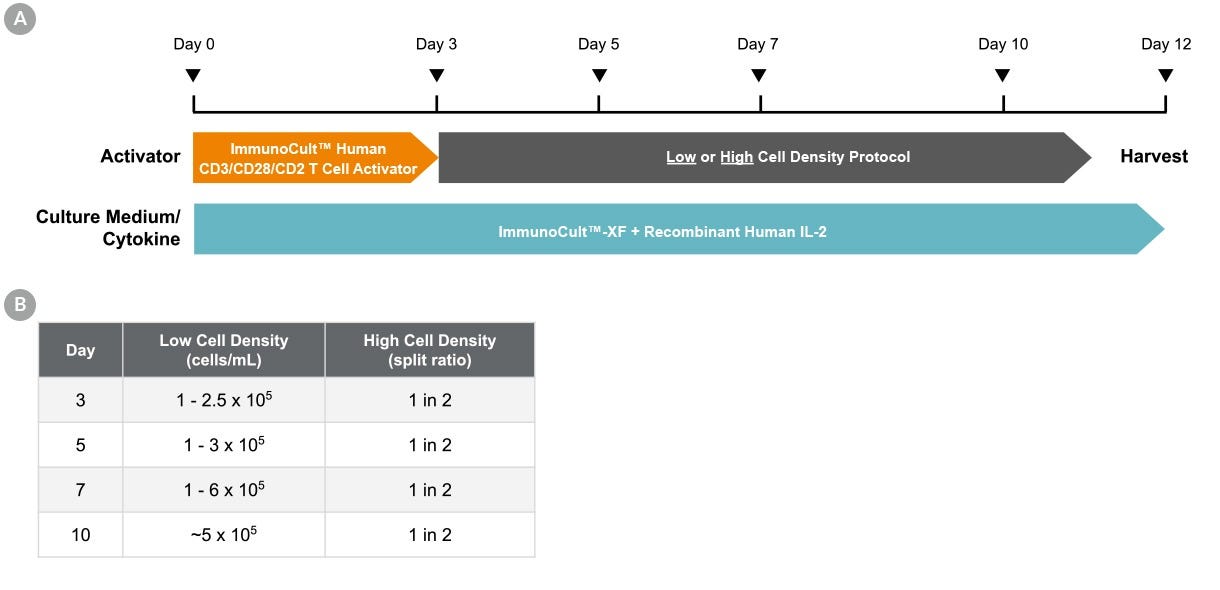
Figure 1. Culture Protocol for T Cell Activation And Expansion
(A) T cells were isolated from fresh leukopaks (Catalog #70500) from healthy donors using EasySep™ Human T Cell Isolation Kit (Catalog #17951) and cryopreserved in CryoStor® CS10 (Catalog #07930). On Day 0, T cells were thawed, washed, and resuspended at 1 x 10⁶ cells/mL in ImmunoCult™-XF (Catalog #100-0956) supplemented with 180 IU/mL Human Recombinant IL-2, ACF (rhIL-2; Catalog #78145). Cells were seeded into 24-well tissue culture plates (1 mL/well) and stimulated with 25 µL/mL ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator (Catalog #100-0785)*. On Days 3, 5, 7, and 10, cells were re-seeded in fresh ImmunoCult™-XF + rhIL-2 at low or high cell densities as outlined in the table (B). Cells were harvested on Day 12.
*A similar protocol was performed using ImmunoCult™ Human CD3/CD28 T Cell Activator (Catalog #100-0784). Please reach out to our Product and Scientific Support team for additional information.
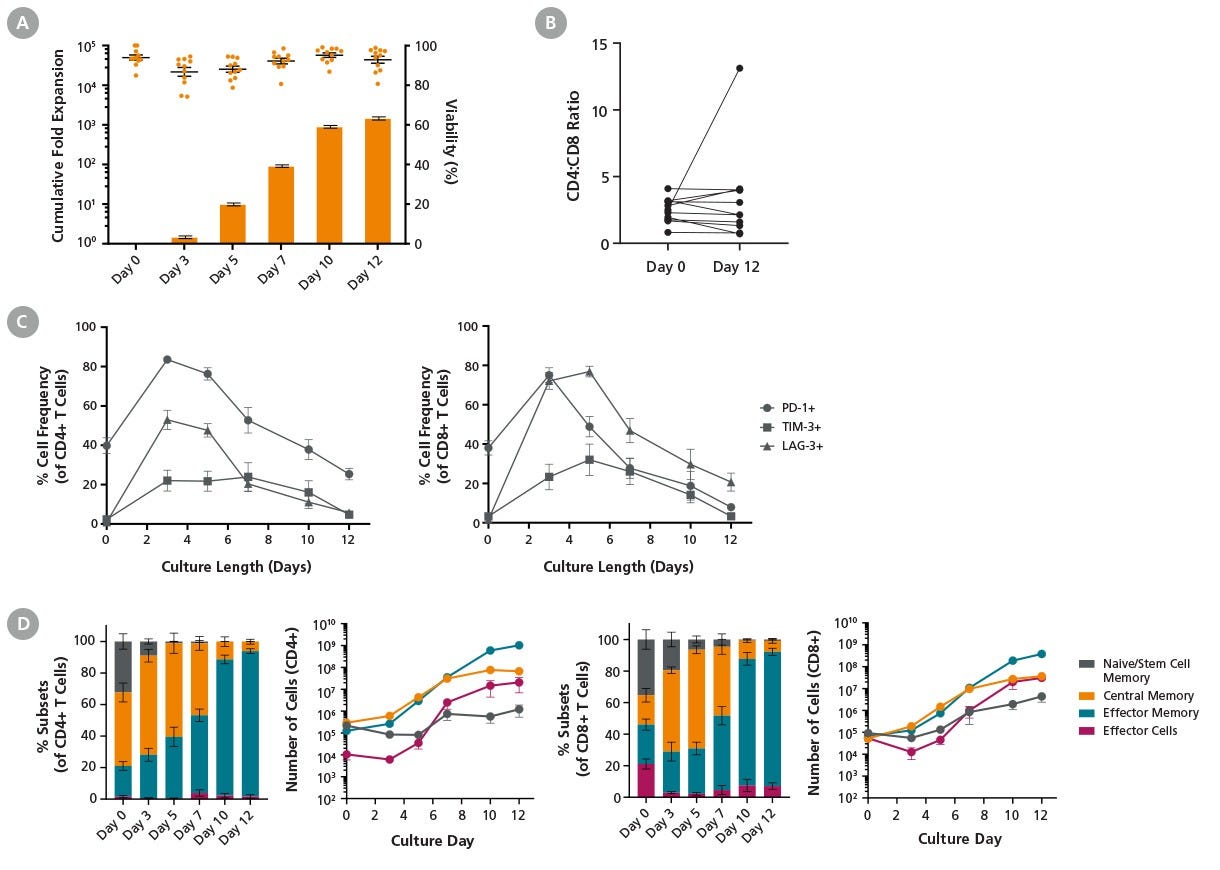
Figure 2. ImmunoCult™ Reagents Result in Robust T Cell Expansion Under Low Cell Density Culture Conditions
T cells were cultured with ImmunoCult™-XF (Catalog #100-0956), Human Recombinant IL-2, ACF (Catalog #78145), and ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator (Catalog #100-0785) for 12 days following the low cell density protocol described in Figure 1. Cells were harvested and analyzed at different time points. Cell count and viability were assessed using the NucleoCounter® NC-250™ automated cell analyzer, and cell surface marker expression was analyzed by flow cytometry.
(A) Points and bars in the graph represent T cell viability and average cumulative fold expansion, respectively, for each assessed culture time point. An average viability of 92.8 ± 1.8% and a total fold expansion of 1497 ± 143 were achieved by Day 12 in the low cell density cultures. Data represent mean ± SEM (n = 11).
(B) The ratio of CD4+ T cells to CD8+ T cells (CD4:CD8) was comparable between Day 0 and Day 12 in 10 out of 11 donors.
(C) The frequency of T cells expressing PD-1, TIM-3, and LAG-3 was upregulated upon stimulation and subsequently decreased to basal levels (n = 4).
(D) The frequency and number of T cell subtypes in CD4+ and CD8+ populations during the 12-day culture period are shown. Cell numbers were calculated based on 10⁶ starting T cells. The number of central memory T cells remained constant from day 7 to 12. T cell subsets: naive and T memory stem cell (TN/SCM) CCR7+CD45RO-; central memory (TCM) CCR7+CD45RO+; effector memory (TEM) CCR7-CD45RO+; effector cells (TEFF) CCR7-CD45RO- (n = 4 - 11).
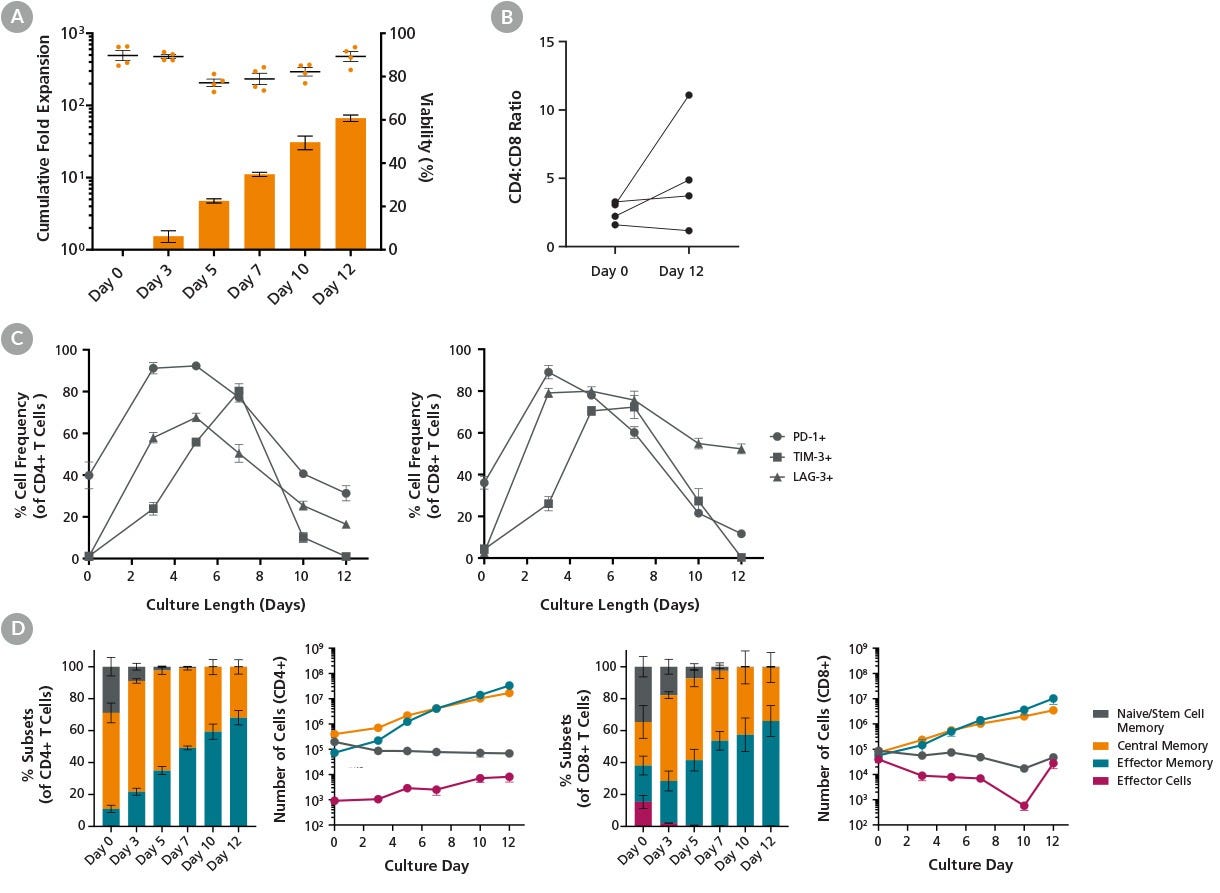
Figure 3. ImmunoCult™ Reagents Support T Cell Activation and Expansion Following High Cell Density Protocol
T cells were cultured with ImmunoCult™-XF (Catalog #100-0956), Human Recombinant IL-2, ACF (Catalog #78145), and ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator (Catalog #100-0785) for 12 days following the high cell density protocol described in Figure 1. Cells were harvested at different time points and analyzed. Cell count and viability were assessed using the NucleoCounter® NC-250™ automated cell analyzer, and cell surface marker expression was analyzed by flow cytometry.
(A) Points and bars in the graph represent T cell viability and average cumulative fold expansion, respectively, for each assessed culture time point. An average viability of 89.3 ± 2.3% and a total fold expansion of 67 ± 7 were achieved by Day 12 in the high cell density cultures (mean ± SEM, n = 4).
(B) The ratio of CD4+ T cells to CD8+ T cells (CD4:CD8) was measured at the beginning and end of culture. The CD4:CD8 ratios increased in two out of four donors tested.
(C) The frequency of T cells expressing PD-1, TIM-3, and LAG-3 increased upon stimulation and subsequently decreased to basal levels by Day 12 of culture (n = 4), with the exception of LAG-3 expression in the CD8⁺ population.
(D) Data shows the frequency and number of T cell subtypes in CD4+ and CD8+ populations at the indicated time points. Cell numbers were calculated based on 10⁶ starting T cells. The number of central memory T cells continued to increase from Day 7 to Day 12 of culture. T cell subsets: naive and T memory stem cells (TN/SCM) CCR7+CD45RO-; central memory (TCM) CCR7+CD45RO+; effector memory (TEM) CCR7-CD45RO+; effector cells (TEFF) CCR7-CD45RO- (n = 4).
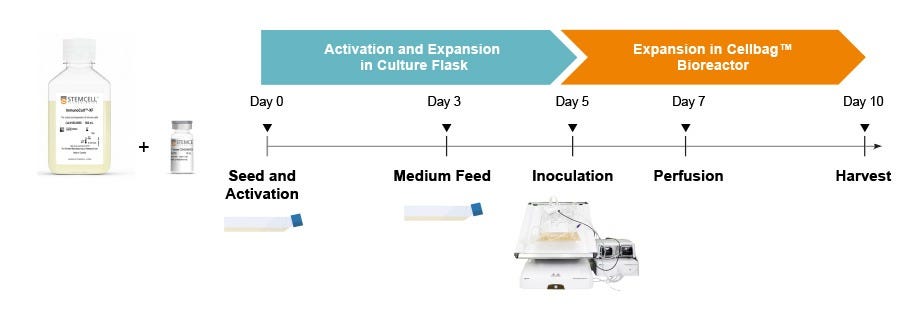
Figure 4. Culture Protocol for Expansion of T Cells with ImmunoCult™ Reagents in the Xuri™ Cell Expansion System W2
T cell expansion workflow in Xuri™ Cell Expansion System W25: T cells were isolated from fresh leukopaks (Catalog #70500) using the EasySep™ Human T Cell Isolation Kit (Catalog #17951) and cryopreserved in Cryostor® CS10 (Catalog #07930). On Day 0, cryopreserved cells were thawed and resuspended at 1 x 10⁶ cells/mL in ImmunoCult™-XF (Catalog #100-0956) supplemented with 180 IU/mL Human Recombinant IL-2, ACF (Catalog #78145). 5 x 10⁷ T cells were seeded in a T175 tissue culture flask and activated with ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator (Catalog #100-0785) at 25 μL/mL. On Day 3, cell density was adjusted to 1 - 2.5 x 10⁵ cells/mL by adding fresh ImmunoCult™-XF with IL-2. On Day 5, the cell suspension containing 3 x 10⁸ cells/L of culture volume was inoculated into a Xuri™ 2L Cellbag™ Bioreactor and cultured until Day 10. Once the cell density reached 1.5 x 10⁶ cells/mL, perfusion was initiated at 0.5 L/day on Day 7 and increased to 1 L/day on Day 9. The culture was harvested on Day 10.
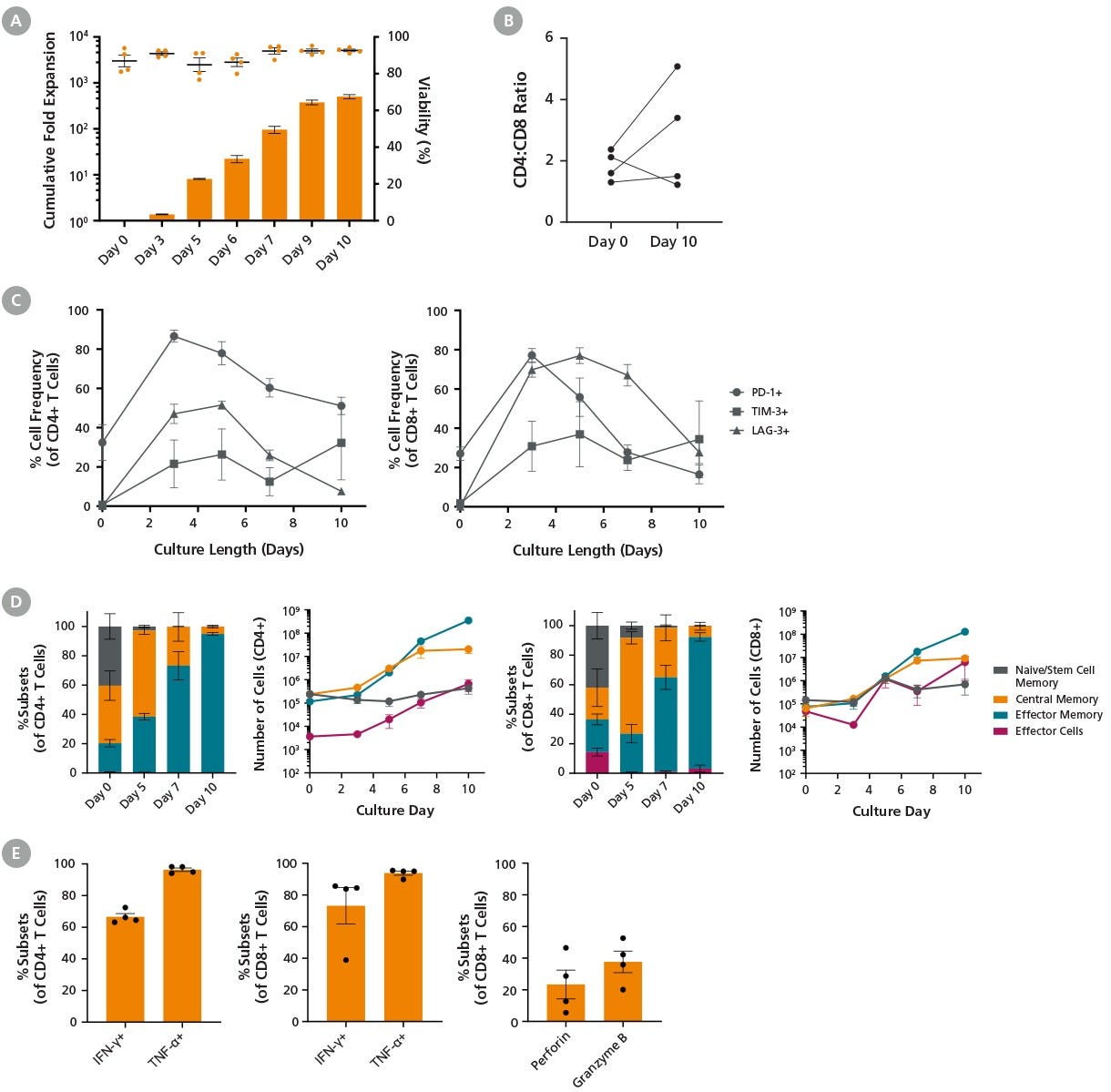
Figure 5. Expansion of T Cells with ImmunoCult™ Reagents in the Xuri™ Cell Expansion System W25
T cells were cultured with ImmunoCult™-XF (Catalog #100-0956), Human Recombinant IL-2, ACF (Catalog #78145), and ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator (Catalog #100-0785) for 10 days following the protocol described in Figure 4.
(A) Points and bars in the graph represent T cell viability and average cumulative fold expansion, respectively, for each assessed culture time point. On average, 92.7 ± 0.7% viability and a total fold expansion of 503 ± 51 were achieved by Day 10 (mean ± SEM, n=4).
(B) Changes in the ratio of CD4+ T cells to CD8+ T cells (CD4:CD8) were measured at the beginning and end of culture. Some variability among donors was observed (n = 4).
(C) The frequency of T cells expressing PD-1, TIM-3, and LAG-3 increased by Day 3 and subsequently decreased from Day 3 to Day 10 (n = 4).
(D) The frequency and number of T cell subtypes in CD4+ and CD8+ populations are shown. Cell numbers were calculated based on 10⁶ starting T cells. The number of central memory T cells peaked on Day 7 of the culture. T cell subsets: naive and T memory stem cells (TN/SCM) CCR7+CD45RO-; central memory (TCM) CCR7+CD45RO+; effector memory (TEM) CCR7-CD45RO+; effector cells (TEFF) CCR7-CD45RO- (n = 4).
(E) The frequencies of expanded T cells expressing IFN-ɣ and TNF-ɑ upon stimulation with PMA and ionomycin were 66.6 ± 2.1% and 96.3 ± 1.1%, respectively, in the CD4+ population, and 73.2 ± 11.4% and 93.8 ± 1.3%, in the CD8⁺ population. Additionally, the frequencies of expanded CD8+ T cells expressing perforin and granzyme B without stimulation were 23.3 ± 9.1% and 37.7 ± 6.8%, respectively (n = 4).
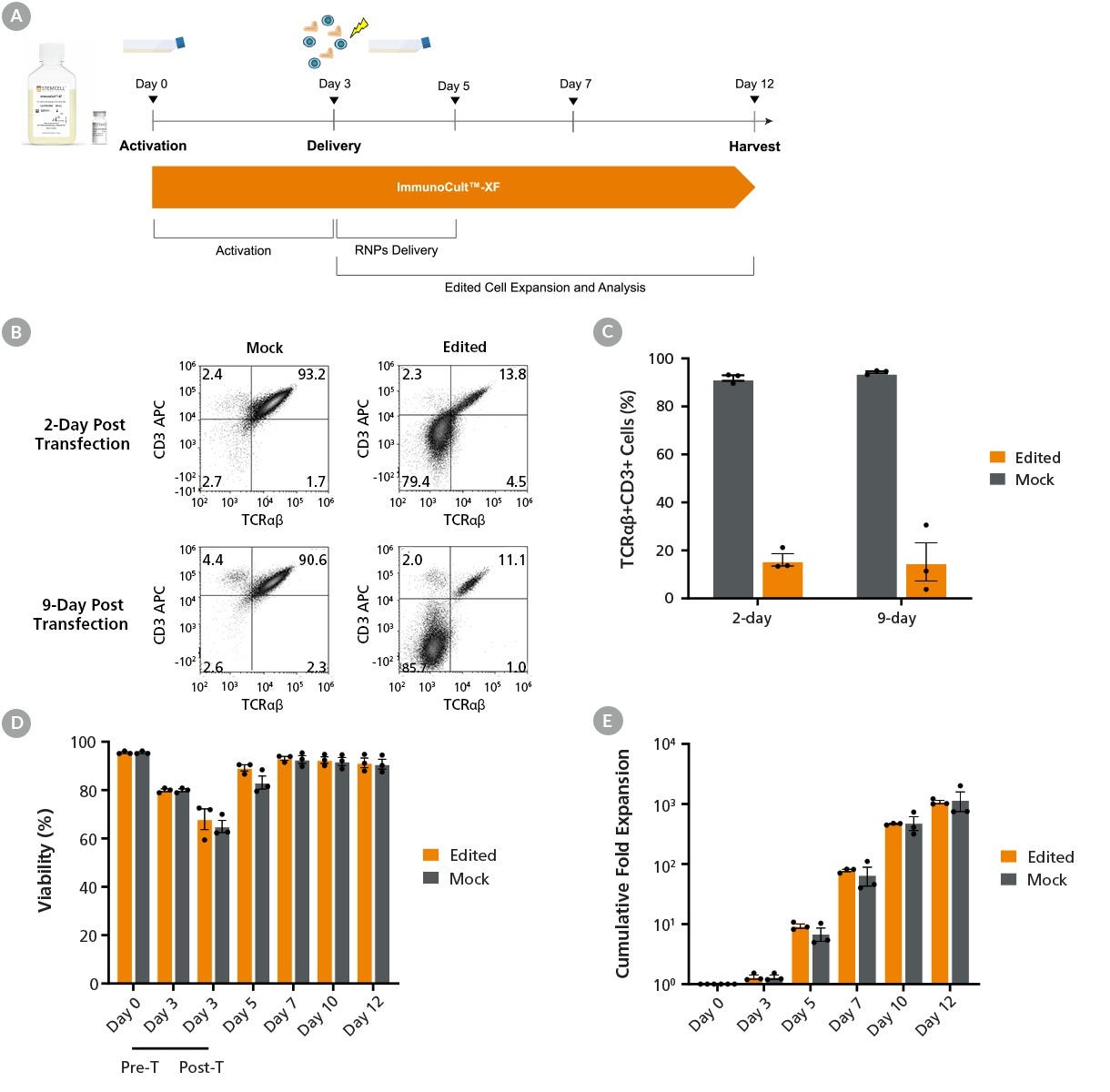
Figure 6. High-Efficiency TRAC Knockout of T Cells
(A) The protocol for non-viral genetic modification and expansion of T cells: On Day 0, T cells were isolated from fresh leukopaks using the EasySep™ Human T Cell Isolation Kit (Catalog #17951). Cells were resuspended at 1 x 10⁶ cells/mL in ImmunoCult™-XF (Catalog #100-0956) supplemented with 180 IU/mL Human Recombinant IL-2, ACF (rhIL-2; Catalog #78145). Cells were seeded into 24-well tissue culture plates at 1 mL/well and stimulated with ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator (Catalog #100-0785) at 25 μL/mL. Following 3 days of activation, RNP complexes containing ArciTect™ Cas9 Nuclease (Catalog #76004) and sgRNA targeting the TRAC gene were delivered to T cells by electroporation (Neon® Transfection System). Cells were then returned to culture in ImmunoCult™-XF supplemented with rhIL-2 for another 9 days of expansion following the low cell density protocol.
(B) Representative flow cytometry plots of TCRαβ and CD3 expression analysis on Days 2 and 9 post-T cell editing.
(C) The gene editing efficiency was assessed by measuring the frequency of the remaining TCRαβ+CD3+ cells in the mock (91.8 ± 1.1%) and edited T cells (16.1 ± 2.6%) 2 days post-editing. The frequency of TRAC-edited cells was maintained throughout the culture duration.
(D) The viability of T cells was measured using the NucleoCounter® NC-250™ automated cell analyzer across culture periods. On Day 3, the viability of T cells further decreased immediately following transfection.
(E) Cumulative cell expansion post-electroporation was comparable between mock-treated and TRAC-edited T cells, suggesting that the edited T cells are functional. Data represent mean ± SEM (n = 3).
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Resources and Publications
Educational Materials (13)
Related Products
-
 CryoStor® CS10
CryoStor® CS10Animal component-free, defined cryopreservation medium with 10% DMSO
-
 ImmunoCult™ Human CD3/CD28 T Cell Activator
ImmunoCult™ Human CD3/CD28 T Cell ActivatorcGMP, human T cell activation and expansion reagent
-
 ImmunoCult™ Human CD3/CD28/CD2 T Cell Activ...
ImmunoCult™ Human CD3/CD28/CD2 T Cell Activ...cGMP, human T cell activation and expansion reagent
-
 EasySep™ Human T Cell Isolation Kit
EasySep™ Human T Cell Isolation KitImmunomagnetic negative isolation of untouched human T cells
-
 Human Recombinant IL-2 (CHO-expressed)
Human Recombinant IL-2 (CHO-expressed)Interleukin 2
Item added to your cart

ImmunoCult™-XF
THIS PRODUCT IS MANUFACTURED AND TESTED FOLLOWING RELEVANT CGMPs UNDER A CERTIFIED QUALITY MANAGEMENT SYSTEM. PRODUCT IS FOR FURTHER MANUFACTURING OR RESEARCH USE. NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.














