Enzyme-Free Passaging of Human Pluripotent Stem Cells Using ReLeSR™
How to passage ES and iPS cells cultured in mTeSR™ Plus using ReLeSR™
In cell culture, the process of transferring cultured cells to fresh growth medium is known as passaging. A number of different methods may be used to passage cells enzymatically or non-enzymatically, depending on the cell culture medium and matrix being used. For example, human embryonic stem (ES) and induced pluripotent stem (iPS) cells maintained in mTeSR™ Plus on Vitronectin XF™ are recommended to be passaged with non-enzymatic reagents, such as ReLeSR™. As an enzyme-free dissociation reagent, ReLeSR™ enables passaging of human ES and iPS cells as aggregates without manual selection of differentiated areas or scraping to remove cell aggregates*.
The following protocol describes how to passage ES and iPS cells cultured in mTeSR™ Plus using ReLeSR™. These instructions are for passaging cells from one well of a 6-well plate. If using other cultureware, adjust volumes accordingly. Please consult your Product Information Sheet for recommendations on suitable passaging reagents and methods for your cell culture system.
*Note: If manual selection of differentiated areas is required, see this Protocol for Passaging Using Gentle Cell Dissociation Reagent.
Materials
- Cell culture matrix (e.g. Vitronectin XF™, Catalog #07180 or Corning® Matrigel®)
- mTeSR™ Plus (Catalog #05825)
- Note: This protocol can also be used for ES and iPS cells cultured in mTeSR™1 (Catalog #85850) or TeSR™-E8™ (Catalog #05990)
- ReLeSR™ (Catalog #05872)
- 6-well plate (e.g. Flat-Bottom Plates, Non-Treated, Catalog #38040)
- D-PBS (Without Ca++ and Mg++; Catalog #37350)
Protocol
- At least 1 hour before passaging, coat new plates with either Vitronectin XF™ or Corning® Matrigel® (see section 4.2.1 of the Technical Manual: Maintenance of Human Pluripotent Stem Cells in mTeSR™ Plus).
- Aliquot sufficient mTeSR™ Plus and warm to room temperature (15 - 25°C).
Note: Do not warm mTeSR™ Plus in a 37°C water bath. - Wash cells with 1 mL of D-PBS (Without Ca++ and Mg++) and aspirate.
Note: There is no need to remove regions of differentiated cells. - Add 1 mL of ReLeSR™ and aspirate ReLeSR™ within 1 minute, so that colonies are exposed to a thin film of liquid.
- Incubate at 37°C for 6 - 8 minutes.
Note: Optimal dissociation time may vary depending on the cell line used; when passaging a cell line with ReLeSR™ for the first time, the optimal dissociation time should be determined (for more information see Figure 1 and consult our Troubleshooting Guide for hPSC Culture).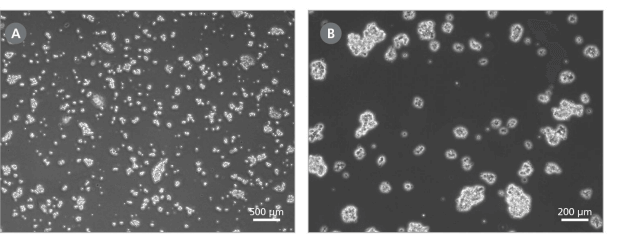
Figure 1. Effect of ReLeSR™ on Human Pluripotent Cells
Examples of ideal cell aggregates (mean size of approximately 50 - 200 μm) obtained after step 8 of the ReLeSR™ protocol. Magnifications: (A) 40X and (B) 100X. If cell aggregates do not resemble these examples, the passaging protocol may require further optimization.
- Add 1 mL of mTeSR™ Plus.
- Place the plate on a plate vortexer at 1200 rpm for 2 - 3 minutes at room temperature. Alternatively, hold the plate with one hand and use the other hand to firmly tap the side of the plate for approximately 30 - 60 seconds.
- Transfer the detached cell aggregates to a 15 mL conical tube using a 5 mL serological pipette. Cell aggregates should be appropriately sized for plating (mean aggregate size of approximately 50 - 200 μm; see Figure 1).
Note: If you wish to plate cell aggregates directly from your passaged well (i.e. without transferring into a tube), pipette the aggregate mixture up and down once using a 5 mL pipette. This will ensure breakup of any large aggregates that may still be present. - Plate the cell aggregate mixture at the desired density onto coated wells containing mTeSR™ Plus. If the colonies are at an optimal density, the cultures can be split every 4 - 7 days using 1 in 10 to 1 in 50 splits (i.e. cell aggregates from 1 well can be plated in 10 to 50 wells). If the colonies are too dense or too sparse, adjust the split ratio accordingly at the next time of passaging (see section 6.4 of the Technical Manual: Maintenance of Human Pluripotent Stem Cells in mTeSR™ Plus.
- Place the plate in a 37°C incubator. Move the plate in several quick, short, back-and-forth and side-to-side motions to evenly distribute the cell aggregates. Do not disturb the plate for 24 hours.
Note: Uneven distribution of aggregates may result in increased differentiation of human ES and iPS cells. - Perform medium changes as needed using mTeSR™ Plus and visually assess cultures to monitor growth until the next passaging time. Medium can be changed daily or every other day. To skip two consecutive days of feeding, add twice the volume of medium.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration