How to Generate AssemBloids™ from hPSC-Derived Dorsal and Ventral Forebrain Organoid Co-Cultures
Patterned neural organoids corresponding to particular brain regions can be generated from human pluripotent stem cells (hPSCs).1 For a more complex system, organoids patterned to different regions of the brain may be merged together in co-culture, generating AssemBloids™.2 These merged, functionally integrated organoid systems can be used to study interneuron migration and neural circuit connectivity. The following protocol is for generating AssemBloids™ by combining the two types of neural organoids patterned using STEMdiff™ Dorsal Forebrain Organoid and STEMdiff™ Ventral Forebrain Organoid Differentiation Kits concurrently.
- We recommend using dorsal and ventral forebrain organoids between 30 and 100 days old. The optimal age within this range is dependent on the downstream assay and should be determined by the user.
- If organoids are between 30 - 43 days old when initiating this protocol, STEMdiff™ Neural Organoid Supplement C (Component #08625) must be included in the AssemBloids™ co-culture medium prepared in step 2.
- To confirm the brain-region origin of interneurons or other cells of interest, a GFP reporter line such as GFP-Dlxi1/22 or GFP-WA01 (H1) ES cells3 may be used in the differentiation step of one of the brain region types from hPSCs.
Materials
- Ultra-low attachment 96-well round-bottom microplate (Corning Catalog #7007)
- The following components of STEMdiff™ Dorsal Forebrain Organoid Differentiation Kit (Catalog #08620):
- STEMdiff™ Neural Organoid Basal Medium 2 (Component #08622)*
- STEMdiff™ Neural Organoid Supplement A (Component #08623)*
- STEMdiff™ Neural Organoid Supplement C (Component #08625)**
- 1000-μL pipette tips (e.g. Corning® Filtered Pipette Tips, Catalog #38031)
- 1000-μL pipettor (e.g. Corning® Lambda™ Plus Pipettor, Catalog #38058)
*Components #08622 and #08623 are available for purchase together as the STEMdiff™ Neural Organoid Maintenance Kit (Catalog #100-0120).
**Component #08625 (Supplement C) is only required if organoids are used between Day 30 - 43. If this option is chosen, ensure that excess Supplement C (or excess Forebrain Organoid Differentiation Medium consisting of Basal Medium 2, Supplement A, and Supplement C) is saved from the earlier stages of the STEMdiff™ Dorsal and Ventral Forebrain Organoid protocols.
Protocol
This procedure has been optimized for use with hPSC maintenance reagents and multiple embryonic stem (ES) and induced pluripotent stem (iPS) cell lines. For upstream protocols and source materials, please see the mTeSR™ Plus Technical Manual and the Product Information Sheet for STEMCELL’s highly quality-controlled Healthy Control Human iPSC Line, Female, SCTi003-A.
- Generate dorsal and ventral forebrain organoids by following the protocol in the Product Information Sheet for STEMdiff™ Dorsal and Ventral Forebrain Organoid Differentiation Kits (Document #10000007462). Continue the protocol until at least Day 30 for each type of organoid before proceeding to step 2.
- Prepare complete AssemBloids™ co-culture medium as follows:
a. Combine 98 mL of STEMdiff™ Neural Organoid Basal Medium 2 and 2 mL of STEMdiff™ Neural Organoid Supplement A.Note: STEMdiff™ Neural Organoid Basal Medium 2 is viscous; pipette slowly to ensure medium is transferred effectively.b. Optional: If using organoids between the ages of Day 30 - 43, also add 0.1 mL of STEMdiff™ Neural Organoid Supplement C. If organoids are more than 43 days old when initiating this protocol, Supplement C is not required.
c. Mix thoroughly and warm to room temperature before use.
d. If not used immediately, store the complete medium at 2 - 8°C for up to 3 weeks. Do not exceed the shelf life of the basal medium or supplements. - Add 200 µL of AssemBloids™ co-culture medium to one well of an ultra-low attachment 96-well plate.
- Cut a 1000 μL pipette tip to generate a wide-bore tip, and use it to transfer one dorsal forebrain organoid followed by one ventral forebrain organoid to the well containing medium (prepared in step 3).
- Using a microscope, confirm that the two organoids are settled at the bottom of the well and in contact with each other. If the organoids are not touching, gently tap the plate until they are.
- Incubate the plate at 37°C and 5% CO2.
- After 24 hours, observe the organoids to determine if they have merged.
Note: Merging of the organoids may take 48 - 72 hours. If desired, AssemBloids™ may be transferred from the 96-well plate into a 24-well plate once they have merged. Carefully transfer individual AssemBloids™ using a cut 1000 μL pipette tip into separate wells of a 24-well plate containing 500 μL of AssemBloids™ Co-Culture Medium per well.
- 72 hours (3 days) after combining the organoids, perform a half-medium change, as follows: Carefully remove half of the medium, then add back the same volume of fresh AssemBloids™ co-culture medium. Continue to perform half-medium changes every 2 - 3 days. AssemBloids™ have been successfully cultured for as long as 24 days in this way.2
Note: Larger volumes and/or more frequent medium changes may be required if older organoids are used or if the medium is being used up more quickly (i.e. medium turns yellow quickly).
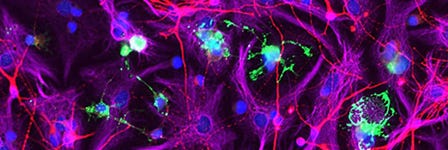
- Interneuron migration, an indicator of functional integration of the AssemBloids™, can typically be observed 5 - 7 days after merging of the organoids. For information on live imaging conditions for AssemBloids™ co-cultures using BrainPhys™ Imaging Optimized Medium (Catalog #05796), please see Zabolocki et al.3 Interneurons may be characterized by the expression of NKX2.1, GAD1, vGAT, and Dlxi1/2b, which can be assessed by fixation and immunofluorescence with appropriate antibodies.
See neural organoid cryopreservation and section immunofluorescence technical bulletin >Note: To confirm that these interneurons arose from the ventral forebrain organoid, a GFP reporter line may be used (see Important Protocol Notes above).
- Sloan et al. (2018) Generation and assembly of human brain region-specific three-dimensional cultures. Nat Protoc 13(9): 2062–85.
- Birey F et al. (2017) Assembly of functionally integrated human forebrain spheroids. Nature 545(7652): 54–9.
- Zabolocki M et al. (2020) BrainPhys neuronal medium optimized for imaging and optogenetics in vitro. Nat Comm 11(1): 5550.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration