Data Quality and Standards for Pluripotent Stem Cells
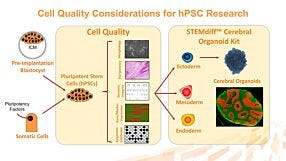
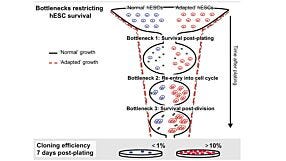
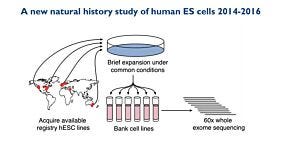
In this recorded webinar, Dr. Andreas Kurtz from the BCRT discusses the data and quality standards around human pluripotent stem research including cell line accessibility, ethical considerations, and more.
In this recorded webinar, Dr. Andreas Kurtz discusses the data and quality standards around human pluripotent stem research including cell line accessibility, ethical considerations, and more. Dr. Kurtz is a steering committee member of the International Stem Cell Banking Initiative (ISCBI) and is founding PI of the Berlin-Brandenburg Center for Regenerative Therapies at the Charité in Berlin. Since 2010 he has managed hPSCreg, a freely accessible global registry for human hPSC lines.
Publish Date:
September 28, 2018
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
By submitting this form, you are providing your consent to STEMCELL Technologies Canada Inc. and its subsidiaries and affiliates (“STEMCELL”) to collect and use your information, and send you newsletters and emails in accordance with our privacy policy. Please contact us with any questions that you may have. You can unsubscribe or change your email preferences at any time.