hPSC Quality: Essential Considerations for Gene Editing, Cloning, Maintenance and Disease Modeling
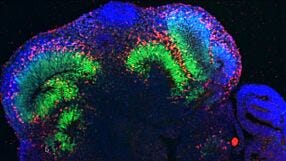
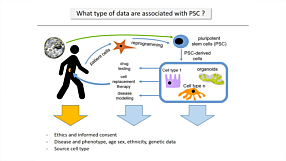
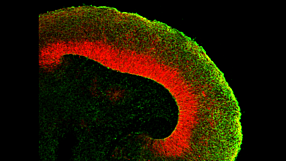
With CRISPR-Cas9 technology, generation of genome-edited human pluripotent cell lines has increased. This seminar discusses critical quality control aspects for robust hPSC cultures, exemplified with disease modeling methods using cerebral organoids.
Publish Date:
July 13, 2018
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
By submitting this form, you are providing your consent to STEMCELL Technologies Canada Inc. and its subsidiaries and affiliates (“STEMCELL”) to collect and use your information, and send you newsletters and emails in accordance with our privacy policy. Please contact us with any questions that you may have. You can unsubscribe or change your email preferences at any time.